Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 4B - IBD / Obesity / Stomach / Pediatrics
72 - Comparative Effectiveness of Upadacitinib vs Ustekinumab for Ulcerative Colitis at 8-16 Weeks: A Multicenter Retrospective Cohort Study
Wednesday, October 25, 2023
9:40 AM - 9:50 AM PT
Location: Ballroom B
- RD
Rahul Dalal, MD, MPH (he/him/his)
Brigham and Women's Hospital, Harvard Medical School
Boston, MA
Presenting Author(s)
Award: Outstanding Research Award in the IBD Category (Trainee)
Rahul Dalal, MD, MPH1, Govind Kallumkal, MD2, Heidy Cabral, BS, MD1, Salam Bachour, MD, MS1, Edward Barnes, MD, MPH, FACG3, Jessica R.. Allegretti, MD, MPH1
1Brigham and Women's Hospital, Harvard Medical School, Boston, MA; 2University of North Carolina School of Medicine, Chapel Hill, NC; 3University of North Carolina at Chapel Hill, Chapel Hill, NC
Introduction: We sought to compare the effectiveness of upadacitinib (Upa), a novel JAK-1 inhibitor, to ustekinumab (Uste) for ulcerative colitis (UC) at 8-16 weeks.
Methods: In this retrospective cohort study, adults initiated Upa or Uste for UC between 1/1/2021-2/1/2023 at two large academic centers. Electronic records were manually reviewed. Patients with Crohn’s disease, prior colectomy, and treatment for non-UC indications were excluded. The primary outcome was clinical response at 8-16 weeks, defined using the following tiered criteria based on data availability: A. improvement in simple clinical colitis activity index (SCCAI) by >3 pts or B. improvement in 9-pt Mayo score by >3 pts or C. provider documentation of clinical response. Secondary outcomes were steroid-free clinical remission (SFCR) at 8-16 weeks (no steroid requirements and A. SCCAI < 2, or B. Mayo <2, or C. provider documentation of clinical remission), endoscopic response (improvement in Mayo endoscopic subscore [MES] by >1 pt), and endoscopic remission (MES=0) at first endoscopic assessment within 52 weeks. Other outcomes: biochemical remission (C-reactive protein < 10 mg/L), improvement in arthralgia (if present), and discontinuation within 16 weeks. Drug-related adverse events (AEs) were reported descriptively. Inverse probability of treatment-weighted (IPTW) logistic regression was performed to determine the association of Upa vs Uste with primary/secondary outcomes.
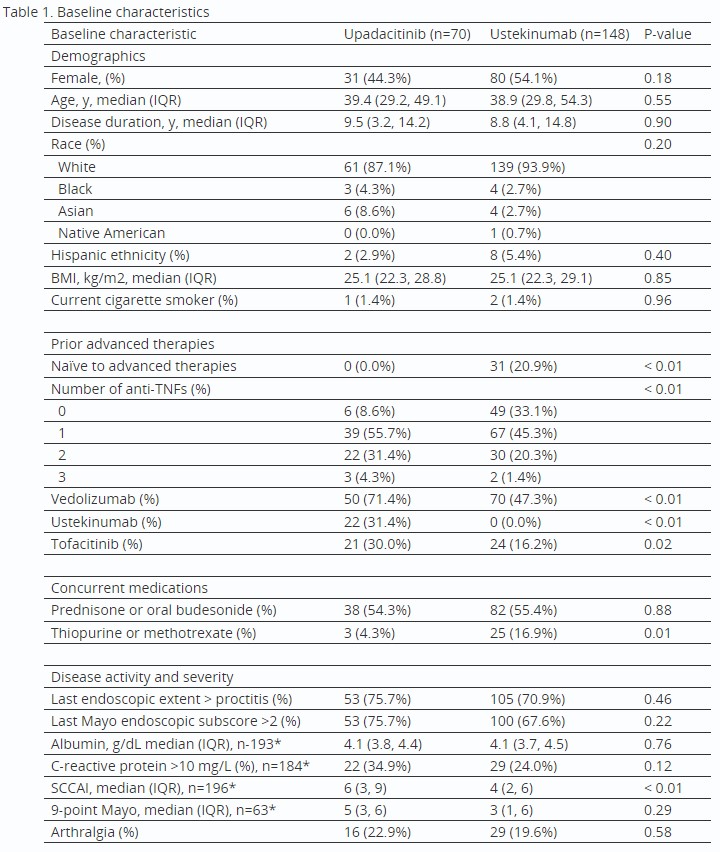
Results: The cohort included 218 unique patients (70 Upa, 148 Uste). Upa patients had more prior advanced therapy failures and higher baseline SCCAI (Table 1). A higher proportion of Upa patients met all outcomes except for treatment discontinuation and endoscopic response, which were similar between groups (Fig 1A). AEs reported within 16 weeks for Upa (n=5) included acne, angina due to anemia, elevated liver enzymes, nausea, and pneumonia and for Uste (n=10) included appendicitis, arthralgia, acute infusion reaction, bone pain, bowel microperforation, fatigue, nausea, and rash (n=4). After IPTW, which successfully balanced covariates (Fig 1B), Upa was associated with significantly higher odds of clinical response (OR 2.39), SFCR (OR 3.72), and endoscopic remission (OR 5.10) vs Uste (Fig 1C). Results were similar in a sensitivity analysis that excluded Upa patients with prior Uste exposure (n=22) (Fig 1C).
Discussion: In a real-world UC cohort, Upa was associated with significantly higher odds of clinical response, SFCR, and endoscopic remission vs Uste.

Disclosures:
Rahul Dalal, MD, MPH1, Govind Kallumkal, MD2, Heidy Cabral, BS, MD1, Salam Bachour, MD, MS1, Edward Barnes, MD, MPH, FACG3, Jessica R.. Allegretti, MD, MPH1, 72, Comparative Effectiveness of Upadacitinib vs Ustekinumab for Ulcerative Colitis at 8-16 Weeks: A Multicenter Retrospective Cohort Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
Rahul Dalal, MD, MPH1, Govind Kallumkal, MD2, Heidy Cabral, BS, MD1, Salam Bachour, MD, MS1, Edward Barnes, MD, MPH, FACG3, Jessica R.. Allegretti, MD, MPH1
1Brigham and Women's Hospital, Harvard Medical School, Boston, MA; 2University of North Carolina School of Medicine, Chapel Hill, NC; 3University of North Carolina at Chapel Hill, Chapel Hill, NC
Introduction: We sought to compare the effectiveness of upadacitinib (Upa), a novel JAK-1 inhibitor, to ustekinumab (Uste) for ulcerative colitis (UC) at 8-16 weeks.
Methods: In this retrospective cohort study, adults initiated Upa or Uste for UC between 1/1/2021-2/1/2023 at two large academic centers. Electronic records were manually reviewed. Patients with Crohn’s disease, prior colectomy, and treatment for non-UC indications were excluded. The primary outcome was clinical response at 8-16 weeks, defined using the following tiered criteria based on data availability: A. improvement in simple clinical colitis activity index (SCCAI) by >3 pts or B. improvement in 9-pt Mayo score by >3 pts or C. provider documentation of clinical response. Secondary outcomes were steroid-free clinical remission (SFCR) at 8-16 weeks (no steroid requirements and A. SCCAI < 2, or B. Mayo <2, or C. provider documentation of clinical remission), endoscopic response (improvement in Mayo endoscopic subscore [MES] by >1 pt), and endoscopic remission (MES=0) at first endoscopic assessment within 52 weeks. Other outcomes: biochemical remission (C-reactive protein < 10 mg/L), improvement in arthralgia (if present), and discontinuation within 16 weeks. Drug-related adverse events (AEs) were reported descriptively. Inverse probability of treatment-weighted (IPTW) logistic regression was performed to determine the association of Upa vs Uste with primary/secondary outcomes.
Results: The cohort included 218 unique patients (70 Upa, 148 Uste). Upa patients had more prior advanced therapy failures and higher baseline SCCAI (Table 1). A higher proportion of Upa patients met all outcomes except for treatment discontinuation and endoscopic response, which were similar between groups (Fig 1A). AEs reported within 16 weeks for Upa (n=5) included acne, angina due to anemia, elevated liver enzymes, nausea, and pneumonia and for Uste (n=10) included appendicitis, arthralgia, acute infusion reaction, bone pain, bowel microperforation, fatigue, nausea, and rash (n=4). After IPTW, which successfully balanced covariates (Fig 1B), Upa was associated with significantly higher odds of clinical response (OR 2.39), SFCR (OR 3.72), and endoscopic remission (OR 5.10) vs Uste (Fig 1C). Results were similar in a sensitivity analysis that excluded Upa patients with prior Uste exposure (n=22) (Fig 1C).
Discussion: In a real-world UC cohort, Upa was associated with significantly higher odds of clinical response, SFCR, and endoscopic remission vs Uste.

Figure: Figure 1. A. Unadjusted outcomes of upadacitinib vs ustekinumab. The first four outcomes were assessed at first follow-up between 8-16 weeks after treatment initiation. Treatment discontinuation was assessed within 16 weeks and endoscopic outcomes were assessed at first endoscopic evaluation within 52 weeks. B. Standardized mean differences of covariates before and after IPTW. C. Results of IPTW logistic regression for primary and secondary outcomes. Sensitivity analysis excludes upadacitinib patients with prior ustekinumab exposure.
Abbreviations: IPTW = inverse probability of treatment weighting, aOR = adjusted odds ratio, CI = confidence interval
Abbreviations: IPTW = inverse probability of treatment weighting, aOR = adjusted odds ratio, CI = confidence interval

Table: *Data not available for full cohort for these variables. Albumin, C-reactive protein, SCCAI, and 9-point Mayo scores were included only if documented within 3 months prior to treatment initiation.
Disclosures:
Rahul Dalal: Centaur Labs – Consultant. Janssen – Consultant, Grant/Research Support. Pfizer – Grant/Research Support.
Govind Kallumkal indicated no relevant financial relationships.
Heidy Cabral indicated no relevant financial relationships.
Salam Bachour indicated no relevant financial relationships.
Edward Barnes: AbbVie, Inc. – Consultant. Bristol-Meyers Squibb – Consultant. Eli Lilly – Consultant. Target RWE – Consultant.
Jessica Allegretti: Abbvie – Consultant, Speakers Bureau. Adiso Therapeutics – Consultant. Artizan – Consultant. Artugen Therapeutics – Consultant. Baccain – Consultant. Bristol-Myers Squibb/Celgene – Consultant, Speakers Bureau. Ferring Pharmaceuticals – Consultant. Finch Therapeutics – Consultant. Iterative Scopes – Consultant. Janssen – Consultant, Grant/Research Support, Speakers Bureau. Merck – Consultant, Grant/Research Support. Morphic – Consultant. Pandion Therapeutics – Consultant. Pfizer – Consultant, Grant/Research Support. Roivant Sciences – Consultant. Seres Therapeutics – Consultant. Servatus – Consultant. Summit – Consultant.
Rahul Dalal, MD, MPH1, Govind Kallumkal, MD2, Heidy Cabral, BS, MD1, Salam Bachour, MD, MS1, Edward Barnes, MD, MPH, FACG3, Jessica R.. Allegretti, MD, MPH1, 72, Comparative Effectiveness of Upadacitinib vs Ustekinumab for Ulcerative Colitis at 8-16 Weeks: A Multicenter Retrospective Cohort Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.


