Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 4B - IBD / Obesity / Stomach / Pediatrics
69 - Efficacy of Guselkumab in Patients With Moderately to Severely Active Crohn’s Disease Not in Clinical Response at Week 12: Results From the GALAXI 1 Study
Wednesday, October 25, 2023
9:10 AM – 9:20 AM PT
Location: Ballroom B
.jpg)
Anita Afzali, MD, MPH, MHCM, FACG
Executive Vice Chair, Department of Internal Medicine
University of Cincinnati
Cincinnati, OH
Presenting Author(s)
Remo Panaccione, MD1, Anita Afzali, MD, MPH, FACG2, Geert R. D’Haens, PhD3, Leonardo Salese, MD4, Natalie A. Terry, MD4, Aparna Sahoo, DO4, Mary Ellen Frustaci, PhD4, Zijiang Yang, PhD4, Eran Zittan, MD5, Silvio Danese, MD, PhD6, Tadakazu Hisamatsu, MD, PhD7
1University of Calgary, Calgary, AB, Canada; 2University of Cincinnati College of Medicine, Cincinnati, OH; 3Amsterdam University Medical Center, Amsterdam, Drenthe, Netherlands; 4Janssen Research & Development, LLC, Spring House, PA; 5Emek Medical Center, Afula, HaDarom, Israel; 6IRCCS San Raffaele Scientific Institute, Vita-Salute San Raffaele University, Milan, Lombardia, Italy; 7Kyorin University School of Medicine, Tokyo, Tokyo, Japan
Introduction: The Phase 2 GALAXI 1 study evaluated the efficacy and safety of guselkumab (GUS), a human selective IL-23p19 subunit antagonist, in patients (pts) with moderate to severe Crohn’s disease (CD). Pts were randomized to GUS, ustekinumab (UST), or placebo (PBO) through Week (Wk) 48 without rerandomization after IV induction. Previously, we reported that pts who were in clinical response after IV induction had higher rates of clinical and endoscopic outcomes at Wk48 than the overall study population.1 In this post hoc analysis, we report outcomes for pts who did not achieve Wk12 clinical response based on CD activity index (CDAI) after IV induction.
Methods: Pts were randomized 1:1:1:1:1 to induction with GUS 200, 600, or 1200mg IV, UST ∼6mg/kg IV, or PBO IV. At Wk12 (Wk8 for UST), pts were transitioned to maintenance dosing: GUS 200mg IV→100mg subcutaneous (SC) q8w, GUS 600mg IV→200mg SC q4w, GUS 1200mg IV→200mg SC q4w, UST ∼6mg/kg IV→90mg SC q8w, PBO nonresponders→UST ∼6mg/kg IV→90mg SC q8w, and PBO responders→PBO SC q4w. Clinical response (all time points) was defined as ≥100-point reduction from baseline in CDAI or CDAI < 150. Other endpoints included clinical remission (CDAI < 150), pt-reported outcome (PRO)-2 remission (unweighted CDAI components of average daily abdominal pain score ≤1 and average daily stool frequency ≤3, and no worsening from baseline), and endoscopic response (≥50% improvement from baseline in simple endoscopic score [SES]-CD or SES-CD ≤2). The study was not powered to evaluate between-group efficacy differences at Wk48; UST was used for reference.
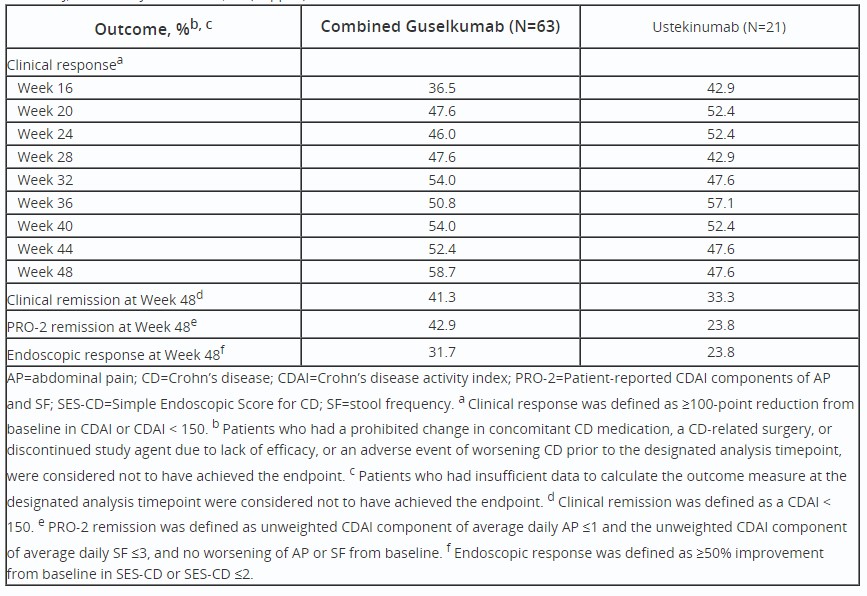
Results: At Wk12, 63/185 (34.1%) GUS pts and 21/63 (33.3%) UST did not achieve CDAI clinical response. Among GUS Wk12 nonresponders, 46.0% were in clinical response by Wk24 (Table). Percentages of Wk12 nonresponders who went on to achieve outcomes at Wk48 in the GUS and UST groups, respectively, were as follows: clinical response, 58.7% and 47.6%; clinical remission, 41.3% and 33.3%; PRO-2 remission, 42.9% and 23.8%; and endoscopic response, 31.7% and 23.8% (Table).
Discussion: Among pts who were not in clinical response at Wk12, continued treatment with GUS SC may result in clinical response and, ultimately, clinical or PRO-2 remission and/or endoscopic response.
1. Panés J, et al. UEG Journal 2022; 10 (Suppl 8).
Disclosures:
Remo Panaccione, MD1, Anita Afzali, MD, MPH, FACG2, Geert R. D’Haens, PhD3, Leonardo Salese, MD4, Natalie A. Terry, MD4, Aparna Sahoo, DO4, Mary Ellen Frustaci, PhD4, Zijiang Yang, PhD4, Eran Zittan, MD5, Silvio Danese, MD, PhD6, Tadakazu Hisamatsu, MD, PhD7, 69, Efficacy of Guselkumab in Patients With Moderately to Severely Active Crohn’s Disease Not in Clinical Response at Week 12: Results From the GALAXI 1 Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of Calgary, Calgary, AB, Canada; 2University of Cincinnati College of Medicine, Cincinnati, OH; 3Amsterdam University Medical Center, Amsterdam, Drenthe, Netherlands; 4Janssen Research & Development, LLC, Spring House, PA; 5Emek Medical Center, Afula, HaDarom, Israel; 6IRCCS San Raffaele Scientific Institute, Vita-Salute San Raffaele University, Milan, Lombardia, Italy; 7Kyorin University School of Medicine, Tokyo, Tokyo, Japan
Introduction: The Phase 2 GALAXI 1 study evaluated the efficacy and safety of guselkumab (GUS), a human selective IL-23p19 subunit antagonist, in patients (pts) with moderate to severe Crohn’s disease (CD). Pts were randomized to GUS, ustekinumab (UST), or placebo (PBO) through Week (Wk) 48 without rerandomization after IV induction. Previously, we reported that pts who were in clinical response after IV induction had higher rates of clinical and endoscopic outcomes at Wk48 than the overall study population.1 In this post hoc analysis, we report outcomes for pts who did not achieve Wk12 clinical response based on CD activity index (CDAI) after IV induction.
Methods: Pts were randomized 1:1:1:1:1 to induction with GUS 200, 600, or 1200mg IV, UST ∼6mg/kg IV, or PBO IV. At Wk12 (Wk8 for UST), pts were transitioned to maintenance dosing: GUS 200mg IV→100mg subcutaneous (SC) q8w, GUS 600mg IV→200mg SC q4w, GUS 1200mg IV→200mg SC q4w, UST ∼6mg/kg IV→90mg SC q8w, PBO nonresponders→UST ∼6mg/kg IV→90mg SC q8w, and PBO responders→PBO SC q4w. Clinical response (all time points) was defined as ≥100-point reduction from baseline in CDAI or CDAI < 150. Other endpoints included clinical remission (CDAI < 150), pt-reported outcome (PRO)-2 remission (unweighted CDAI components of average daily abdominal pain score ≤1 and average daily stool frequency ≤3, and no worsening from baseline), and endoscopic response (≥50% improvement from baseline in simple endoscopic score [SES]-CD or SES-CD ≤2). The study was not powered to evaluate between-group efficacy differences at Wk48; UST was used for reference.
Results: At Wk12, 63/185 (34.1%) GUS pts and 21/63 (33.3%) UST did not achieve CDAI clinical response. Among GUS Wk12 nonresponders, 46.0% were in clinical response by Wk24 (Table). Percentages of Wk12 nonresponders who went on to achieve outcomes at Wk48 in the GUS and UST groups, respectively, were as follows: clinical response, 58.7% and 47.6%; clinical remission, 41.3% and 33.3%; PRO-2 remission, 42.9% and 23.8%; and endoscopic response, 31.7% and 23.8% (Table).
Discussion: Among pts who were not in clinical response at Wk12, continued treatment with GUS SC may result in clinical response and, ultimately, clinical or PRO-2 remission and/or endoscopic response.
1. Panés J, et al. UEG Journal 2022; 10 (Suppl 8).

Table: Table. Efficacy Outcomes Among Patients Not in Clinical Response(a) at Week 12.
Disclosures:
Remo Panaccione: Abbivax – Consultant. Abbott – Consultant. AbbVie – Consultant. Alimentiv – Consultant. Amgen – Consultant. Arena – Consultant. AstraZeneca – Consultant. Biogen – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. Celltrion – Consultant. Cosmos Technology – Consultant. Eisai – Consultant. Elan – Consultant. Eli Lilly – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Galapagos – Consultant. Genentech – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. JAMP Bio – Consultant. Janssen – Consultant. Merck – Consultant. Mylan – Consultant. Novartis – Consultant. Oppilan Pharma – Consultant. Organon – Consultant. Pandion Pharma – Consultant. Pendopharm – Consultant. Pfizer Inc – Consultant. Progenity – Consultant. Prometheus – Consultant. Protagonist Therapeutics – Consultant. Roche – Consultant. Sandoz – Consultant. Satisfai Health – Consultant. Shire – Consultant. Sublimity Therapeutics – Consultant. Takeda Pharmaceuticals – Consultant. Theravance Biopharma – Consultant. Trellus – Consultant. UCB – Consultant. Ventyx – Consultant. Viatris – Consultant.
Anita Afzali: AbbVie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Bristol Myers Squibb/Celgene – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. DiaSorin – Consultant. Eli Lilly – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Gilead – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. IBD Horizons – Owner/Ownership Interest. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Takeda – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. TLL Pharmaceuticals – Consultant.
Geert D’Haens: AbbVie – Advisor or Review Panel Member, Speakers Bureau. Agomab Therapeutics – Advisor or Review Panel Member. Alimentiv – Advisor or Review Panel Member. Applied Molecular Therapeutics – Advisor or Review Panel Member. AstraZeneca – Advisor or Review Panel Member. Boehringer Ingelheim – Advisor or Review Panel Member. Bristol Myers Squibb – Advisor or Review Panel Member, Speakers Bureau. Celltrion – Advisor or Review Panel Member. Cytoki – Advisor or Review Panel Member. Eli Lilly – Advisor or Review Panel Member, Speakers Bureau. Exeliom – Advisor or Review Panel Member. Ferring – Advisor or Review Panel Member. Galapagos – Advisor or Review Panel Member, Speakers Bureau. GlaxoSmithKline – Advisor or Review Panel Member. Gossamer Bio – Advisor or Review Panel Member. Immunic – Advisor or Review Panel Member. Johnson & Johnson – Advisor or Review Panel Member, Speakers Bureau. Pfizer – Advisor or Review Panel Member, Speakers Bureau. Polpharma – Advisor or Review Panel Member. ProciseDx – Advisor or Review Panel Member. Progenity – Advisor or Review Panel Member. Prometheus Biosciences – Advisor or Review Panel Member. Prometheus Laboratories – Advisor or Review Panel Member. Protagonist Therapeutics – Advisor or Review Panel Member. Seres – Advisor or Review Panel Member. Takeda – Advisor or Review Panel Member, Speakers Bureau. Tillotts – Advisor or Review Panel Member, Speakers Bureau. Versant – Advisor or Review Panel Member.
Leonardo Salese: Janssen – Employee.
Natalie Terry: Janssen – Employee.
Aparna Sahoo: Janssen – Employee.
Mary Ellen Frustaci: Janssen – Employee.
Zijiang Yang: Janssen – Employee.
Eran Zittan: AbbVie – Consultant, Grant/Research Support. Celgene – Consultant, Grant/Research Support. Janssen – Consultant, Grant/Research Support. Neopharm – Consultant, Grant/Research Support. Pfizer – Consultant, Grant/Research Support. Takeda – Consultant, Grant/Research Support.
Silvio Danese: AbbVie – Consultant, personal fees (lecture fees). Alimentiv – Consultant. Allergan – Consultant, personal fees. Amgen – Consultant, lecture fees. Applied Molecular Transport – Consultant. AstraZeneca – Consultant, personal fees. Athos Therapeutics – Consultant, personal fees. Biogen – Consultant, personal fees. Boehringer Ingelheim – Consultant, personal fees. Bristol Myers Squibb – Consultant. Celgene – Consultant, personal fees. Celltrion Healthcare – Consultant, Personal fees. Dr Falk Pharma – Consultant. Eli Lilly – Consultant, personal fees. Enthera – Consultant, personal fees. Ferring Pharmaceuticals – Consultant, lecture fees. Gilead – Consultant, lecture fees. Hospira – Consultant, personal fees. Inotrem – Consultant, personal fees. Janssen Pharmaceuticals – Consultant, lecture fees. Johnson & Johnson – Consultant, personal fees. Morphic – Consultant. MSD – Consultant, personal fees. Mundipharma – Consultant, personal fees. Mylan – Consultant, lecture fees. Pfizer – Consultant, lecture fees. Roche – Consultant, personal fees. Sandoz – Consultant, personal fees. Sublimity Therapeutics – Consultant, personal fees. Takeda – Consultant, lecture fees. Teladoc Health – Consultant. TiGenix – Consultant, personal fees. UCB – Consultant, personal fees. Vial – Consultant. Vifor – Consultant, personal fees.
Tadakazu Hisamatsu: AbbVie GK – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Alfresa Pharma Corporation and EA Pharma Co., Ltd – Grant/Research Support. Daiichi-Sankyo – Grant/Research Support. EA Pharma Co, Ltd – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Eli Lilly – Consultant. Gilead Sciences – honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Janssen Pharmaceutical K.K. – honoraria and had expenses paid to attend or give a presentation/advice at a meeting. JIMRO Co., Ltd – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. KISSEI PHARMACEUTICAL CO., LTD – honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Kyorin Pharmaceutical Co., Ltd – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Mitsubishi Tanabe Pharma Corporation – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Mochida Pharmacuetical Co., Ltd – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Nichi-Iko Pharmaceutical Co., Ltd – honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Nippon Kayaku Co., Ltd – Grant/Research Support. Pfizer Japan Inc. – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Takeda Pharmaceutical Co., Ltd – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Zeria Pharmaceutical Co., Ltd – Grant/Research Support.
Remo Panaccione, MD1, Anita Afzali, MD, MPH, FACG2, Geert R. D’Haens, PhD3, Leonardo Salese, MD4, Natalie A. Terry, MD4, Aparna Sahoo, DO4, Mary Ellen Frustaci, PhD4, Zijiang Yang, PhD4, Eran Zittan, MD5, Silvio Danese, MD, PhD6, Tadakazu Hisamatsu, MD, PhD7, 69, Efficacy of Guselkumab in Patients With Moderately to Severely Active Crohn’s Disease Not in Clinical Response at Week 12: Results From the GALAXI 1 Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

