Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 4B - IBD / Obesity / Stomach / Pediatrics
66 - Metoclopramide Use in the Setting of Upper Gastrointestinal Bleed-An Updated Meta-Analysis
Wednesday, October 25, 2023
8:40 AM - 8:50 AM PT
Location: Ballroom B

Diego A. Reymunde Duran, MD
University of Texas Health Science Center at Houston
Houston, TX
Presenting Author(s)
Diego A.. Reymunde Duran, MD1, Maryam R.. Hussain, 2, Faisal S.. Ali, MD1, Asmeen Bhatt, MD, PhD1
1University of Texas Health Science Center at Houston, Houston, TX; 2School of Public and Population Health University of Texas Medical Branch, Galveston, TX
Introduction: Metoclopramide is commonly used prior to endoscopy in the setting of upper gastrointestinal bleeding (UGIB) to improve endoscopic visualization. Guidance on its use remains limited.
Methods: A systematic review of Medline and Embase from inception-2023 was done to identify studies comparing metoclopramide to placebo/control to assess its efficacy in UGIB. Data are summarized and reported descriptively. A meta-analysis of endoscopic visualization score(s) was done.
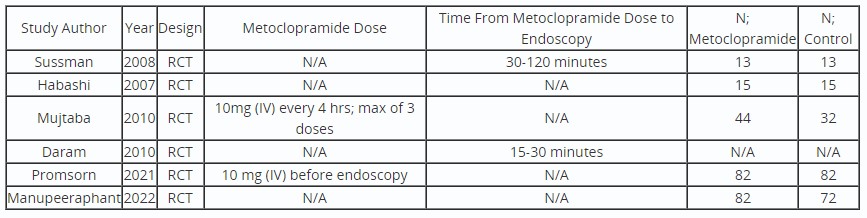
Results: We identified 6 abstracts of RCTs, enrolling 236 patients in the Metoclopramide arm and 214 in the control arm. Only two studies reported the dose and protocol of Metoclopramide use. Between the 6 abstracts, there were multiple endpoints studied including: mean number of RBC transfusion, second look endoscopy, retained clots in the stomach, residual blood in the stomach, endoscopic visualization score, and duration of hospitalization. The mean number of reported RBC transfusions required ranged from 1.34-3.66 in the Metoclopramide and 1.34-2.86 in the control arm. There was no significant difference in the need for second look endoscopy with Metoclopramide use.
Four of the abstracts used the modified Avgerino score (MAS) to quantify endoscopic visualization. Sussman et al. reported better endoscopic visualization in the Metoclopramide arm, however it was not statistically significant. Daram et al. reported no difference in visualization or statistical significance. However, both Promsorn and Manupeeraphant et al. reported improved endoscopic visualization and statistical significance. On comparative meta-analysis, Metoclopramide use was associated with improved endoscopic visualization scores in the antrum (SMD 0.33; 0.11,0.55; I20%), body (SMD 0.30; 0.03,0.58; I236.24%), fundus (SMD 0.94; 0.08,1.80; I292.93%), and total MAS (SMD 0.42; 0.20,0.64; I20%) compared to control.
Discussion: Metoclopramide use is associated with improved endoscopic visualization in the setting of UGIB. Notably, all studies are abstracts of RCTs, and none had a full-text manuscript published. Robustly designed trials that are adequately powered to detect an effect on primary outcomes of interest, particularly endoscopic visualization and residual blood on endoscopy are needed to come to a definitive conclusion. RCTs already conducted should be published beyond abstract form to address evidence gaps.

Disclosures:
Diego A.. Reymunde Duran, MD1, Maryam R.. Hussain, 2, Faisal S.. Ali, MD1, Asmeen Bhatt, MD, PhD1, 66, Metoclopramide Use in the Setting of Upper Gastrointestinal Bleed-An Updated Meta-Analysis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of Texas Health Science Center at Houston, Houston, TX; 2School of Public and Population Health University of Texas Medical Branch, Galveston, TX
Introduction: Metoclopramide is commonly used prior to endoscopy in the setting of upper gastrointestinal bleeding (UGIB) to improve endoscopic visualization. Guidance on its use remains limited.
Methods: A systematic review of Medline and Embase from inception-2023 was done to identify studies comparing metoclopramide to placebo/control to assess its efficacy in UGIB. Data are summarized and reported descriptively. A meta-analysis of endoscopic visualization score(s) was done.
Results: We identified 6 abstracts of RCTs, enrolling 236 patients in the Metoclopramide arm and 214 in the control arm. Only two studies reported the dose and protocol of Metoclopramide use. Between the 6 abstracts, there were multiple endpoints studied including: mean number of RBC transfusion, second look endoscopy, retained clots in the stomach, residual blood in the stomach, endoscopic visualization score, and duration of hospitalization. The mean number of reported RBC transfusions required ranged from 1.34-3.66 in the Metoclopramide and 1.34-2.86 in the control arm. There was no significant difference in the need for second look endoscopy with Metoclopramide use.
Four of the abstracts used the modified Avgerino score (MAS) to quantify endoscopic visualization. Sussman et al. reported better endoscopic visualization in the Metoclopramide arm, however it was not statistically significant. Daram et al. reported no difference in visualization or statistical significance. However, both Promsorn and Manupeeraphant et al. reported improved endoscopic visualization and statistical significance. On comparative meta-analysis, Metoclopramide use was associated with improved endoscopic visualization scores in the antrum (SMD 0.33; 0.11,0.55; I20%), body (SMD 0.30; 0.03,0.58; I236.24%), fundus (SMD 0.94; 0.08,1.80; I292.93%), and total MAS (SMD 0.42; 0.20,0.64; I20%) compared to control.
Discussion: Metoclopramide use is associated with improved endoscopic visualization in the setting of UGIB. Notably, all studies are abstracts of RCTs, and none had a full-text manuscript published. Robustly designed trials that are adequately powered to detect an effect on primary outcomes of interest, particularly endoscopic visualization and residual blood on endoscopy are needed to come to a definitive conclusion. RCTs already conducted should be published beyond abstract form to address evidence gaps.

Figure: Mean Endoscopic Visualization Score-Metoclopramide versus Placebo

Table: Characteristics of Included Studies
*RCT=Randomized Control Trial
*RCT=Randomized Control Trial
Disclosures:
Diego Reymunde Duran indicated no relevant financial relationships.
Maryam Hussain indicated no relevant financial relationships.
Faisal Ali indicated no relevant financial relationships.
Asmeen Bhatt indicated no relevant financial relationships.
Diego A.. Reymunde Duran, MD1, Maryam R.. Hussain, 2, Faisal S.. Ali, MD1, Asmeen Bhatt, MD, PhD1, 66, Metoclopramide Use in the Setting of Upper Gastrointestinal Bleed-An Updated Meta-Analysis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

