Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 2B - Small Intestine / IBD
34 - Early Symptomatic Improvement With Guselkumab Induction Treatment in Moderately to Severely Active Ulcerative Colitis: Results from the Phase 3 QUASAR Induction Study
Tuesday, October 24, 2023
9:30 AM - 9:40 AM PT
Location: Ballroom B

Gary Lichtenstein, MD
University of Pennsylvania
Philadelphia, PA
Presenting Author(s)
Gary R. Lichtenstein, MD1, Axel Dignass, MD, PhD2, David T. Rubin, MD, FACG3, Shadi Yarandi, MD4, Kuan-Hsiang G. Huang, MD, PhD4, Matthew Germinaro, MD4, Ye Miao, MS4, Hongyan Zhang, PhD4, Jaroslaw Kierkuś, MD, PhD5, Ursula Seidler, MD6, Atsuo Maemoto, MD, PhD7, Jessica R.. Allegretti, MD, MPH8, Brian Bressler, MD, MS, FRCPC9, Laurent Peyrin-Biroulet, MD, PhD10
1University of Pennsylvania, Philadelphia, PA; 2Agaplesion Markus Hospital, Goethe University, Frankfurt, Hessen, Germany; 3University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL; 4Janssen Research & Development, LLC, Spring House, PA; 5WIP Warsaw IBD Point, Warsaw, Mazowieckie, Poland; 6Medizinische Hochschule Hannover, Hannover, Niedersachsen, Germany; 7Sapporo Higashi Tokushukai Hospital IBD Center, Sapporo, Hokkaido, Japan; 8Brigham and Women's Hospital, Harvard Medical School, Boston, MA; 9St. Paul’s Hospital, Vancouver, BC, Canada; 10Last Inserm U954 and CHU de Nancy, Lorraine University, Vandoeuvre-lès-Nancy, Lorraine, France
Introduction: The Phase 3 QUASAR Induction Study (NCT04033445) was a randomized, double-blind, placebo-controlled, parallel-group, multicenter study of guselkumab (GUS), an interleukin-23 p19 subunit antagonist, in patients (pts) with moderately to severely active ulcerative colitis (UC). Here the early onset of symptom improvement was evaluated.
Methods: Pts were randomized in a 3:2 ratio to receive IV GUS 200mg or placebo (PBO) at Weeks (Wks) 0, 4, and 8. The primary analysis population included treated pts with a baseline modified Mayo score of 5 to 9, and an endoscopy subscore ≥ 2 (centrally read). Through Wk12, pts recorded stool production and episodes of rectal bleeding in a diary. Symptomatic remission at Wks 2, 4, and 12 were major secondary endpoints. All other analyses were not multiplicity controlled (nominal p-values).
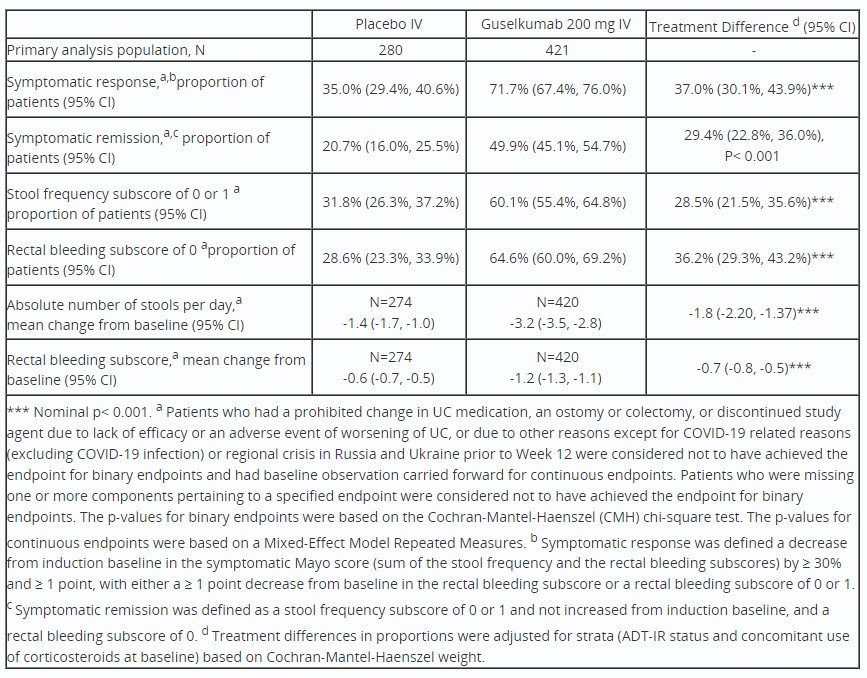
Results: The primary analysis population included 701 randomized and treated pts (mean UC duration, 7.5yrs, Mayo endoscopy subscore=3 [severe disease], 67.9%, and mean modified Mayo score, 6.9, at baseline). At baseline, 49.1% had prior inadequate response/intolerance to advanced therapies (ADT-IR) for UC; nearly half of these (47.4%) had ≥2 ADT-IR classes. At baseline, for the GUS and PBO groups, respectively, mean absolute number of stools per day were 7.10 and 6.96; stool frequency subscores of 0 or 1 were observed in 10.0% and 9.6%; and mean rectal bleeding subscores were 1.7 and 1.8. As early as Wk1 and increasing through Wk12, greater symptomatic improvement was seen in pts treated with GUS compared with PBO (Figure). At Wks 2, 4, 8, and 12, symptomatic remission was achieved by GUS- vs PBO-treated pts in 12.1% vs 9.3%, 22.6% vs 12.9%, 39.7% vs 20.7%, and 49.9% vs 20.7% (all p< 0.001, except Wk2, p=0.210), respectively. For GUS vs PBO, percentages of pts with stool frequency subscore of 0 or 1 at Wks 2, 4, 8, and 12 were 26.1% vs 18.2%, 41.3% vs 25.4%, 53.4% vs 29.6%, and 60.1% vs 31.8% (all p< 0.001, except Wk2, p< 0.05), respectively; percentages of pts with rectal bleeding subscores of 0 at Wks 2, 4, 8, and 12 were 24.2% vs 19.3%, 36.8% vs 22.9%, 55.8% vs 33.2%, and 64.6% vs 28.6% (all p< 0.001, except Wk2, p=0.110), respectively. Treatment differences for GUS vs PBO were evident across Wk12 symptomatic outcomes (Table).
Discussion: GUS 200mg IV induction was effective in improving symptoms as early as 1 week after the first dose in pts with moderately to severely active UC. Symptomatic improvements increased through Wk12.

Disclosures:
Gary R. Lichtenstein, MD1, Axel Dignass, MD, PhD2, David T. Rubin, MD, FACG3, Shadi Yarandi, MD4, Kuan-Hsiang G. Huang, MD, PhD4, Matthew Germinaro, MD4, Ye Miao, MS4, Hongyan Zhang, PhD4, Jaroslaw Kierkuś, MD, PhD5, Ursula Seidler, MD6, Atsuo Maemoto, MD, PhD7, Jessica R.. Allegretti, MD, MPH8, Brian Bressler, MD, MS, FRCPC9, Laurent Peyrin-Biroulet, MD, PhD10, 34, Early Symptomatic Improvement With Guselkumab Induction Treatment in Moderately to Severely Active Ulcerative Colitis: Results from the Phase 3 QUASAR Induction Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of Pennsylvania, Philadelphia, PA; 2Agaplesion Markus Hospital, Goethe University, Frankfurt, Hessen, Germany; 3University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL; 4Janssen Research & Development, LLC, Spring House, PA; 5WIP Warsaw IBD Point, Warsaw, Mazowieckie, Poland; 6Medizinische Hochschule Hannover, Hannover, Niedersachsen, Germany; 7Sapporo Higashi Tokushukai Hospital IBD Center, Sapporo, Hokkaido, Japan; 8Brigham and Women's Hospital, Harvard Medical School, Boston, MA; 9St. Paul’s Hospital, Vancouver, BC, Canada; 10Last Inserm U954 and CHU de Nancy, Lorraine University, Vandoeuvre-lès-Nancy, Lorraine, France
Introduction: The Phase 3 QUASAR Induction Study (NCT04033445) was a randomized, double-blind, placebo-controlled, parallel-group, multicenter study of guselkumab (GUS), an interleukin-23 p19 subunit antagonist, in patients (pts) with moderately to severely active ulcerative colitis (UC). Here the early onset of symptom improvement was evaluated.
Methods: Pts were randomized in a 3:2 ratio to receive IV GUS 200mg or placebo (PBO) at Weeks (Wks) 0, 4, and 8. The primary analysis population included treated pts with a baseline modified Mayo score of 5 to 9, and an endoscopy subscore ≥ 2 (centrally read). Through Wk12, pts recorded stool production and episodes of rectal bleeding in a diary. Symptomatic remission at Wks 2, 4, and 12 were major secondary endpoints. All other analyses were not multiplicity controlled (nominal p-values).
Results: The primary analysis population included 701 randomized and treated pts (mean UC duration, 7.5yrs, Mayo endoscopy subscore=3 [severe disease], 67.9%, and mean modified Mayo score, 6.9, at baseline). At baseline, 49.1% had prior inadequate response/intolerance to advanced therapies (ADT-IR) for UC; nearly half of these (47.4%) had ≥2 ADT-IR classes. At baseline, for the GUS and PBO groups, respectively, mean absolute number of stools per day were 7.10 and 6.96; stool frequency subscores of 0 or 1 were observed in 10.0% and 9.6%; and mean rectal bleeding subscores were 1.7 and 1.8. As early as Wk1 and increasing through Wk12, greater symptomatic improvement was seen in pts treated with GUS compared with PBO (Figure). At Wks 2, 4, 8, and 12, symptomatic remission was achieved by GUS- vs PBO-treated pts in 12.1% vs 9.3%, 22.6% vs 12.9%, 39.7% vs 20.7%, and 49.9% vs 20.7% (all p< 0.001, except Wk2, p=0.210), respectively. For GUS vs PBO, percentages of pts with stool frequency subscore of 0 or 1 at Wks 2, 4, 8, and 12 were 26.1% vs 18.2%, 41.3% vs 25.4%, 53.4% vs 29.6%, and 60.1% vs 31.8% (all p< 0.001, except Wk2, p< 0.05), respectively; percentages of pts with rectal bleeding subscores of 0 at Wks 2, 4, 8, and 12 were 24.2% vs 19.3%, 36.8% vs 22.9%, 55.8% vs 33.2%, and 64.6% vs 28.6% (all p< 0.001, except Wk2, p=0.110), respectively. Treatment differences for GUS vs PBO were evident across Wk12 symptomatic outcomes (Table).
Discussion: GUS 200mg IV induction was effective in improving symptoms as early as 1 week after the first dose in pts with moderately to severely active UC. Symptomatic improvements increased through Wk12.

Figure: Figure. Symptomatic response through Wk12

Table: Table. Symptomatic outcomes at Wk12
Disclosures:
Gary R. Lichtenstein: Abbvie – Consultant. American College of Gastroenterology – Honorarium for Associate Editor of American Journal of Gastroenterology. American Gastroenterological Association – CME. American Regent – Consultant, Honorarium [CME Program]. Celgene – Consultant, Grant/Research Support. CellCeutrix – Consultant. Chemed – CME. Eli Lilly – Consultant, Data Safety Monitoring Board. Endo Pharmaceuticals – Consultant. Ferring – Consultant. Gastroenterology and Hepatology – Gastro-Hep Communication, Editor-Honorarium. Gilead – Consultant. IMEDEX – CME. Ironwood – CME. Janssen/ Janssen Orthobiotech – Consultant, Grant/Research Support, Funding to University of PA [IBD Fellow Education]. MedEd Consultants – Consultant. Merck – Consultant, Honorarium [CME Program]. Morphic Therapeutics – Consultant. Pfizer Pharmaceuticals – Consultant, Funding to University of PA [IBD Fellow Education]. Professional Communications, Inc – Royalty for writing Textbook. Prometheus Laboratories, Inc – Consultant. Romark – Consultant, Honorarium for CME. Salix Pharmaceuticals/Valeant – Consultant. Sandoz – Consultant. Shire Pharmaceuticals – Consultant. SLACK, Inc – Book Royalty. Springer Science and Business Media – Editor [Honorarium]. Takeda – Consultant, Grant/Research Support, Funding to University of PA [IBD Fellow Education]. UCB – Consultant, Grant/Research Support. University of Kentucky – CME. Up-To-Date – Author [Honorarium]. Vindico – CME. Virgo – Consultant, Stock Options.
Axel Dignass: AbbVie – Consultant, participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. Abivax – participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees. Amgen – Consultant. Arena Pharmaceuticals – Consultant, participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees. Biogen – Consultant, Speakers Bureau. Boehringer Ingelheim – Consultant. Bristol Myers Squibb/Celgene – Consultant, participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees. CED Service GmbH – Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Dr Falk Foundation – Consultant, participation in clinical trials, review activities and manuscript preparation, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Fresenius Kabi – Consultant. Galapagos – Consultant, participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. Gilead – participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. High5MD – Speakers Bureau. Janssen – Consultant, participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. Lilly – Consultant. Materia Prima – Speakers Bureau. MedToday – Speakers Bureau. MSD – Consultant, Speakers Bureau. Pfizer – Consultant, participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. Pharmacosmos – Consultant. Roche/Genentech – Consultant. Sandoz/Hexal – Consultant. Streamed-Up – Speakers Bureau. Takeda – Consultant, manuscript preparation, Speakers Bureau. Thieme – manuscript preparation. Tillotts – Consultant, Speakers Bureau. UniMed Verlag – manuscript preparation. Vifor Pharma – Consultant, Speakers Bureau.
David T. Rubin: AbbVie – Consultant. Alike Health – Stock Options. AltruBio – Consultant, Stock Options. Aslan Pharmaceuticals – Consultant. Athos Therapeutics – Consultant. Bellatrix Pharmaceuticals – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. Chronicles – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health, Inc – Advisory Committee/Board Member. Crohn's & Colitis Foundation – Advisory Committee/Board Member. Datos Health – Stock Options. EcoR1 – Consultant. Eli Lilly – Consultant. GastroIntestinal Research Foundation – Grant/Research Support. Genentech/Roche – Consultant. Gilead Sciences – Consultant. Helmsley Charitable Trust – Grant/Research Support. Iterative Health – Consultant. Iterative Health – Stock Options. Janssen – Consultant. Kaleido Biosciences – Consultant. Pfizer – Consultant. Prometheus Biosciences – Consultant. Reistone – Consultant. Seres Therapeutics – Consultant. Syneos – Consultant. Takeda – Consultant, Grant/Research Support. Target RWE – Consultant. Trellus Health – Consultant.
Shadi Yarandi: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Kuan-Hsiang G. Huang: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Matthew Germinaro: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Ye Miao: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Hongyan Zhang: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Jaroslaw Kierkuś: Janssen – Consultant, clinical investigator.
Ursula Seidler: Abbvie – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Abivax – Grant/Research Support. BMS – Grant/Research Support. Eli Lilly – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Galapagos – Advisor or Review Panel Member, Speakers Bureau. Gilead – Grant/Research Support. Janssen – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau.
Atsuo Maemoto: Janssen – Consultant, clinical investigator.
Jessica Allegretti: Abbvie – Consultant, Speakers Bureau. Adiso Therapeutics – Consultant. Artizan – Consultant. Artugen Therapeutics – Consultant. Baccain – Consultant. Bristol-Myers Squibb/Celgene – Consultant, Speakers Bureau. Ferring Pharmaceuticals – Consultant. Finch Therapeutics – Consultant. Iterative Scopes – Consultant. Janssen – Consultant, Grant/Research Support, Speakers Bureau. Merck – Consultant, Grant/Research Support. Morphic – Consultant. Pandion Therapeutics – Consultant. Pfizer – Consultant, Grant/Research Support. Roivant Sciences – Consultant. Seres Therapeutics – Consultant. Servatus – Consultant. Summit – Consultant.
Brian Bressler: AbbVie – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Alimentiv – Advisory Committee/Board Member. Allergan – Advisory Committee/Board Member. Alvine – Grant/Research Support. Amgen – Advisory Committee/Board Member, Grant/Research Support. AMT – Advisory Committee/Board Member. Bausch Health – Advisory Committee/Board Member. Boehringer Ingelheim – Grant/Research Support. Bristol Myers Squibb – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Celgene – Advisory Committee/Board Member, Grant/Research Support. Ferring – Advisory Committee/Board Member, Speakers Bureau. Fresenius Kabi – Advisory Committee/Board Member. Genentech – Advisory Committee/Board Member, Grant/Research Support. Gilead – Advisory Committee/Board Member. GlaxoSmithKline – Grant/Research Support. Iterative Scopes – Advisory Committee/Board Member. Janssen – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Merck – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Microbiome Insights – Advisory Committee/Board Member. Mylan – Advisory Committee/Board Member. Novartis – Advisory Committee/Board Member, Speakers Bureau. Pendopharm – Advisory Committee/Board Member. Pfizer – Advisory Committee/Board Member, Speakers Bureau. Protagonist – Advisory Committee/Board Member. Qu Biologic – Grant/Research Support, Stock Options. Takeda – Advisory Committee/Board Member, Speakers Bureau.
Laurent Peyrin-Biroulet: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Allergan – personal fees. Alma – personal fees. Amgen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Applied Molecular Transport – personal fees. Arena – personal fees. Biogaran – Advisory Committee/Board Member, Consultant, Speakers Bureau. Biogen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant, Speakers Bureau. Bristol Myers Squibb – personal fees. Celgene – Advisory Committee/Board Member, Consultant, Speakers Bureau. Celltrion – Advisory Committee/Board Member, Consultant, Speakers Bureau. CTMA – Stock Options. Eli Lilly – Advisory Committee/Board Member, Consultant, Speakers Bureau. Enterome – personal fees. Enthera – personal fees. Ferring – Advisory Committee/Board Member, Consultant, Speakers Bureau. Forward Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Fresenius – personal fees. Genentech – Advisory Committee/Board Member, Consultant, Speakers Bureau. Gilead – personal fees. H.A.C. Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Hikma – personal fees. Hospira/Pfizer – Advisory Committee/Board Member, Consultant, Speakers Bureau. Index Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speakers Bureau. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Lycera – Advisory Committee/Board Member, Consultant, Speakers Bureau. Merck – Advisory Committee/Board Member, Consultant, Speakers Bureau. Mitsubishi – Advisory Committee/Board Member, Consultant, Speakers Bureau. MSD – Grant/Research Support, personal fees. Mylan – personal fees. Nestlé – personal fees. Norgine – Advisory Committee/Board Member, Consultant, Speakers Bureau. Oppilan Pharma – personal fees. OSE Immunotherapeutics – personal fees. Pfizer – personal fees. Pharmacosmos – personal fees. Roche – personal fees. Samsung Bioepis – Advisory Committee/Board Member, Consultant, Speakers Bureau. Sandoz – Advisory Committee/Board Member, Consultant, Speakers Bureau. Sterna – personal fees. Sublimity Therapeutics – personal fees. Takeda – Advisory Committee/Board Member, Consultant, Speakers Bureau. Theravance – Advisory Committee/Board Member, Consultant, Speakers Bureau. Tillots – Advisory Committee/Board Member, Consultant, Speakers Bureau. Vifor – Advisory Committee/Board Member, Consultant, Speakers Bureau.
Gary R. Lichtenstein, MD1, Axel Dignass, MD, PhD2, David T. Rubin, MD, FACG3, Shadi Yarandi, MD4, Kuan-Hsiang G. Huang, MD, PhD4, Matthew Germinaro, MD4, Ye Miao, MS4, Hongyan Zhang, PhD4, Jaroslaw Kierkuś, MD, PhD5, Ursula Seidler, MD6, Atsuo Maemoto, MD, PhD7, Jessica R.. Allegretti, MD, MPH8, Brian Bressler, MD, MS, FRCPC9, Laurent Peyrin-Biroulet, MD, PhD10, 34, Early Symptomatic Improvement With Guselkumab Induction Treatment in Moderately to Severely Active Ulcerative Colitis: Results from the Phase 3 QUASAR Induction Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

