Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 2A - Functional / Esophagus
25 - Efficacy and Safety of the Selective Sphingosine 1-Phosphate Receptor Modulator, Etrasimod, in Adult Patients With Eosinophilic Esophagitis: Primary Results from the Phase 2 VOYAGE Study
Tuesday, October 24, 2023
9:30 AM - 9:40 AM PT
Location: Ballroom A

Evan S. Dellon, MD, MPH, FACG
Professor of Medicine and Adjunct Professor of Epidemiology, Center for Esophageal Diseases and Swallowing
University of North Carolina at Chapel Hill
Chapel Hill, NC
Presenting Author(s)
Award: Outstanding Research Award in the Esophagus Category
Evan S.. Dellon, MD, MPH1, Margaret H. Collins, MD2, Albert J. Bredenoord, MD3, Hamish Philpott, MBBS, MRCP, PhD4, Luc Biedermann, MD5, Márjori Dulcine, MD, MSc6, Thai Nguyen-Cleary, MD7, Chinyu Su, MD7, Jin Yu, PharmD8, Fabio Cataldi, MD9, Hanzhe Zheng, PhD7, Wenjin Wang, PhD7, Natalie V.. Springveld, MD, PhD10, John C.. Woolcott, PhD7, Ikuo Hirano, MD11
1Center for Esophageal Diseases and Swallowing, and Center for Gastrointestinal Biology and Disease, University of North Carolina School of Medicine, Chapel Hill, NC; 2Cincinnati Children's Hospital Medical Center, Cincinnati, OH; 3Amsterdam University Medical Center, Amsterdam, Noord-Holland, Netherlands; 4Northern Adelaide Local Health Network (NALHN), Lyell McEwin and Modbury Hospitals, University of Adelaide, Elizabeth Vale, Adelaide, South Australia, Australia; 5University Hospital Zürich, Zürich, Zurich, Switzerland; 6Pfizer Brazil, São Paulo, Sao Paulo, Brazil; 7Pfizer Inc., Collegeville, PA; 8Pfizer Inc., Spring House, PA; 9Arena Pharmaceuticals, a wholly-owned subsidiary of Pfizer Inc., San Diego, CA; 10Pfizer AG, Zürich, Zurich, Switzerland; 11Feinberg School of Medicine, Northwestern University, Chicago, IL
Introduction: Etrasimod is an investigational, oral, once-daily (QD), selective sphingosine 1-phosphate (S1P)1,4,5 receptor modulator in development to treat immune-mediated inflammatory disorders. Etrasimod regulates lymphocyte egress from secondary lymphatic organs, and therefore may have promise in EoE (T-cell-mediated disease).
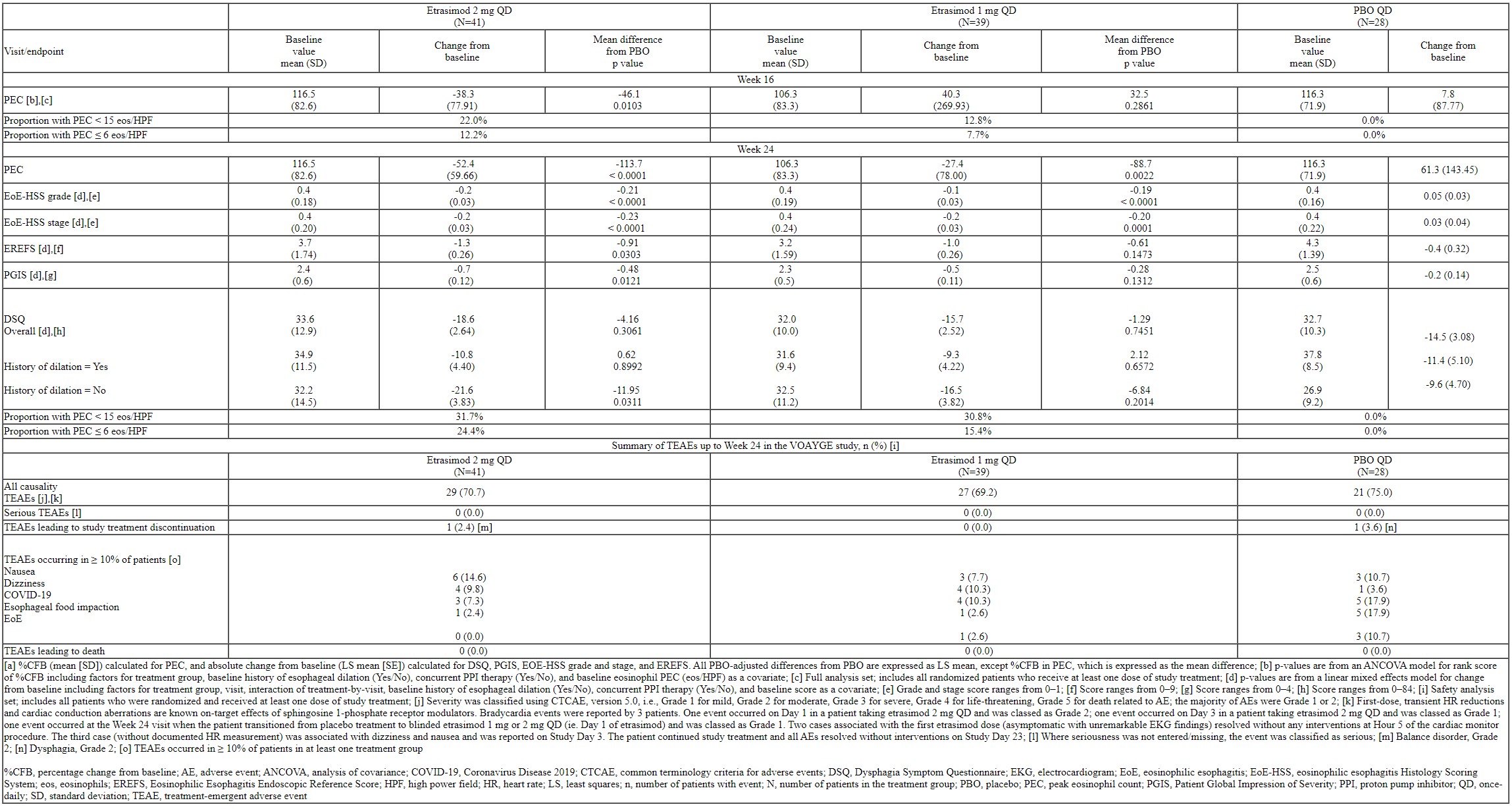
Methods: VOYAGE (NCT04682639) is a phase 2 study with a 24-week (wk) randomized, double-blind, placebo-controlled treatment period, followed by a 28-wk extension period (ongoing), investigating the efficacy and safety of oral etrasimod 1 and 2 mg QD vs placebo (PBO) in adults with active EoE (Figure). The primary efficacy endpoint was the percentage change from baseline (BL) (%CFB) in esophageal peak eosinophil count (PEC) at Wk 16. Other efficacy endpoints assessed the effects of etrasimod on histological and endoscopic features and symptoms of EoE, and included the proportion of pts who achieved PEC < 15 and ≤ 6 eosinophils/high power field, the absolute CFB in EoE-Histology Scoring System (EoE-HSS) grade and stage scores, EoE-Endoscopic Reference Score (EREFS), Patient Global Impression of Severity (PGIS), and Dysphagia Symptom Questionnaire (DSQ) (overall and by BL dilation history) at Wk 24. We present the findings from the double-blind treatment period.
Results: We randomized 108 pts with active EoE to etrasimod 2 mg (N=41), etrasimod 1 mg (N=39) or PBO (N=28) (Figure). In total, 47.2% of pts were female, and at BL, pts had had EoE for an average of 4.6 years with 55.6% having a history of esophageal dilation and 40.0% having prior corticosteroid treatment. Treatment with etrasimod 2 mg QD resulted in a 46.1% decrease from BL in PEC (PBO-adjusted) at Wk 16 (p=0.0103). There were also statistically significant reductions in %CFB in PEC at Wk 24, and reductions in absolute CFB in EoE-HSS grade and stage scores, EREFS, PGIS, and, in pts without BL dilation history, DSQ score (Table). In etrasimod 1 mg- vs PBO-treated pts, there were also statistically significant reductions in %CFB in PEC and absolute CFB in EoE-HSS grade and stage scores at Wk 24. There were no serious treatment-emergent adverse events (TEAEs) reported up to Wk 24; 2 pts discontinued due to TEAEs (Table).
Discussion: Etrasimod 2 mg QD met the primary histologic endpoint and significantly improved endoscopic features of EoE, overall symptom severity, and dysphagia (in pts without dilation history only) at Wk 24, and was well tolerated. This supports further evaluation of etrasimod in EoE.

Disclosures:
Evan S.. Dellon, MD, MPH1, Margaret H. Collins, MD2, Albert J. Bredenoord, MD3, Hamish Philpott, MBBS, MRCP, PhD4, Luc Biedermann, MD5, Márjori Dulcine, MD, MSc6, Thai Nguyen-Cleary, MD7, Chinyu Su, MD7, Jin Yu, PharmD8, Fabio Cataldi, MD9, Hanzhe Zheng, PhD7, Wenjin Wang, PhD7, Natalie V.. Springveld, MD, PhD10, John C.. Woolcott, PhD7, Ikuo Hirano, MD11, 25, Efficacy and Safety of the Selective Sphingosine 1-Phosphate Receptor Modulator, Etrasimod, in Adult Patients With Eosinophilic Esophagitis: Primary Results from the Phase 2 VOYAGE Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
Evan S.. Dellon, MD, MPH1, Margaret H. Collins, MD2, Albert J. Bredenoord, MD3, Hamish Philpott, MBBS, MRCP, PhD4, Luc Biedermann, MD5, Márjori Dulcine, MD, MSc6, Thai Nguyen-Cleary, MD7, Chinyu Su, MD7, Jin Yu, PharmD8, Fabio Cataldi, MD9, Hanzhe Zheng, PhD7, Wenjin Wang, PhD7, Natalie V.. Springveld, MD, PhD10, John C.. Woolcott, PhD7, Ikuo Hirano, MD11
1Center for Esophageal Diseases and Swallowing, and Center for Gastrointestinal Biology and Disease, University of North Carolina School of Medicine, Chapel Hill, NC; 2Cincinnati Children's Hospital Medical Center, Cincinnati, OH; 3Amsterdam University Medical Center, Amsterdam, Noord-Holland, Netherlands; 4Northern Adelaide Local Health Network (NALHN), Lyell McEwin and Modbury Hospitals, University of Adelaide, Elizabeth Vale, Adelaide, South Australia, Australia; 5University Hospital Zürich, Zürich, Zurich, Switzerland; 6Pfizer Brazil, São Paulo, Sao Paulo, Brazil; 7Pfizer Inc., Collegeville, PA; 8Pfizer Inc., Spring House, PA; 9Arena Pharmaceuticals, a wholly-owned subsidiary of Pfizer Inc., San Diego, CA; 10Pfizer AG, Zürich, Zurich, Switzerland; 11Feinberg School of Medicine, Northwestern University, Chicago, IL
Introduction: Etrasimod is an investigational, oral, once-daily (QD), selective sphingosine 1-phosphate (S1P)1,4,5 receptor modulator in development to treat immune-mediated inflammatory disorders. Etrasimod regulates lymphocyte egress from secondary lymphatic organs, and therefore may have promise in EoE (T-cell-mediated disease).
Methods: VOYAGE (NCT04682639) is a phase 2 study with a 24-week (wk) randomized, double-blind, placebo-controlled treatment period, followed by a 28-wk extension period (ongoing), investigating the efficacy and safety of oral etrasimod 1 and 2 mg QD vs placebo (PBO) in adults with active EoE (Figure). The primary efficacy endpoint was the percentage change from baseline (BL) (%CFB) in esophageal peak eosinophil count (PEC) at Wk 16. Other efficacy endpoints assessed the effects of etrasimod on histological and endoscopic features and symptoms of EoE, and included the proportion of pts who achieved PEC < 15 and ≤ 6 eosinophils/high power field, the absolute CFB in EoE-Histology Scoring System (EoE-HSS) grade and stage scores, EoE-Endoscopic Reference Score (EREFS), Patient Global Impression of Severity (PGIS), and Dysphagia Symptom Questionnaire (DSQ) (overall and by BL dilation history) at Wk 24. We present the findings from the double-blind treatment period.
Results: We randomized 108 pts with active EoE to etrasimod 2 mg (N=41), etrasimod 1 mg (N=39) or PBO (N=28) (Figure). In total, 47.2% of pts were female, and at BL, pts had had EoE for an average of 4.6 years with 55.6% having a history of esophageal dilation and 40.0% having prior corticosteroid treatment. Treatment with etrasimod 2 mg QD resulted in a 46.1% decrease from BL in PEC (PBO-adjusted) at Wk 16 (p=0.0103). There were also statistically significant reductions in %CFB in PEC at Wk 24, and reductions in absolute CFB in EoE-HSS grade and stage scores, EREFS, PGIS, and, in pts without BL dilation history, DSQ score (Table). In etrasimod 1 mg- vs PBO-treated pts, there were also statistically significant reductions in %CFB in PEC and absolute CFB in EoE-HSS grade and stage scores at Wk 24. There were no serious treatment-emergent adverse events (TEAEs) reported up to Wk 24; 2 pts discontinued due to TEAEs (Table).
Discussion: Etrasimod 2 mg QD met the primary histologic endpoint and significantly improved endoscopic features of EoE, overall symptom severity, and dysphagia (in pts without dilation history only) at Wk 24, and was well tolerated. This supports further evaluation of etrasimod in EoE.

Figure: Figure. Study Design for VOYAGE. [a] Randomization was stratified by BL history of esophageal dilation and continued use of a stable proton pump inhibitor therapy at study entry; [b] EoE symptom severity and daily dysphagia scores were captured by PGIS and DSQ e-diaries, respectively; esophageal PEC and EoE-HSS (from EGD with biopsy) were centrally read by expert pathologists blinded to treatment assignment. The EREFS was scored by the local endoscopist.
%CFB, percentage change from baseline; BL, baseline; DSQ, Dysphagia Symptom Questionnaire; EGD, esophagogastroduodenoscopy; EoE, eosinophilic esophagitis; EREFS, EoE-Endoscopic Reference Score; EoE-HSS, eosinophilic esophagitis Histology Scoring System; N, number of patients in treatment group; PEC, peak eosinophil count; PGIS, Patient Global Impression of Severity; QD, once-daily; R, randomization
%CFB, percentage change from baseline; BL, baseline; DSQ, Dysphagia Symptom Questionnaire; EGD, esophagogastroduodenoscopy; EoE, eosinophilic esophagitis; EREFS, EoE-Endoscopic Reference Score; EoE-HSS, eosinophilic esophagitis Histology Scoring System; N, number of patients in treatment group; PEC, peak eosinophil count; PGIS, Patient Global Impression of Severity; QD, once-daily; R, randomization

Table: Table. Change from Baseline [a] in PEC at Week 16 (Primary Endpoint), Additional Efficacy Endpoints at Week 24 and a Summary of TEAEs up to Week 24 in the VOYAGE Study.
Disclosures:
Evan Dellon indicated no relevant financial relationships.
Margaret Collins: Allakos – Consultant. AstraZeneca – Consultant. Bristol-Myers Squibb – Consultant. EsoCap – Consultant. Regeneron – Consultant. Sanofi – Consultant. Shire – Consultant.
Albert Bredenoord: Alimentiv – Consultant, Speakers Bureau. Aqilion – Consultant, Speakers Bureau. AstraZeneca – Consultant. Dr. Falk Pharma – Consultant, Grant/Research Support. Eupraxia – Consultant, Speakers Bureau. Laborie – Consultant. Medtronic – Consultant. Norgine – Grant/Research Support. Nutricia – Grant/Research Support. Reckitt – Consultant, Speakers Bureau. Regeneron Pharmaceuticals Inc. – Consultant. Sanofi – Consultant. SST – Grant/Research Support. Thelial – Grant/Research Support.
Hamish Philpott: AusEE – Consultant. Falk – Consultant, Speakers Bureau. GSK – Speakers Bureau. Hospital Research Foundation South Australia – Grant/Research Support.
Luc Biedermann: AbbVie – Advisory Committee/Board Member, Consultant, Speaker's fees. Amgen – Advisory Committee/Board Member. BMS – Advisory Committee/Board Member, Consultant, Speaker's fees. Falk – Advisory Committee/Board Member, Consultant, Speaker's fees. Janseen – Advisory Committee/Board Member. Pfizer – Advisory Committee/Board Member, Consultant, Speaker's fees. Sanofi – Advisory Committee/Board Member, Consultant, Speaker's fees. Takeda – Advisory Committee/Board Member, Consultant, Speaker's fees.
Márjori Dulcine: Pfizer Brazil – Employee. Pfizer Inc – Stock-publicly held company(excluding mutual/index funds).
Thai Nguyen-Cleary: Pfizer Inc – Employee, Stock-publicly held company(excluding mutual/index funds).
Chinyu Su: Pfizer Inc – Employee, Stock-publicly held company(excluding mutual/index funds).
Jin Yu: Pfizer Inc – Employee, Stock-publicly held company(excluding mutual/index funds).
Fabio Cataldi: Arena Pharmaceuticals, a wholly owned subsidiary of Pfizer Inc – Employee.
Hanzhe Zheng: Pfizer Inc – Employee, Stock-publicly held company(excluding mutual/index funds).
Wenjin Wang: Pfizer Inc – Employee, Stock Options.
Natalie Springveld: Pfizer AG – Employee.
John Woolcott: Pfizer Inc – Employee, Stock Options.
Ikuo Hirano: Adare/Ellodi – Consultant, Grant/Research Support. Allakos – Consultant, Grant/Research Support. Amgen – Consultant. Arena/Pfizer – Consultant, Grant/Research Support. Aslan – Consultant. AstraZeneca – Consultant, Grant/Research Support. Celgene/Receptos/Bristol-Myers Squibb – Consultant, Grant/Research Support. Celldex – Consultant. EsoCap – Consultant. Gossamer Bio – Consultant. Meritage – Grant/Research Support. Nexstone Immunology – Consultant. NIH – Grant/Research Support. Parexel/Calyx – Consultant. Phathom – Consultant. Regeneron – Consultant, Grant/Research Support. Sanofi – Consultant. Shire/Takeda – Consultant, Grant/Research Support.
Evan S.. Dellon, MD, MPH1, Margaret H. Collins, MD2, Albert J. Bredenoord, MD3, Hamish Philpott, MBBS, MRCP, PhD4, Luc Biedermann, MD5, Márjori Dulcine, MD, MSc6, Thai Nguyen-Cleary, MD7, Chinyu Su, MD7, Jin Yu, PharmD8, Fabio Cataldi, MD9, Hanzhe Zheng, PhD7, Wenjin Wang, PhD7, Natalie V.. Springveld, MD, PhD10, John C.. Woolcott, PhD7, Ikuo Hirano, MD11, 25, Efficacy and Safety of the Selective Sphingosine 1-Phosphate Receptor Modulator, Etrasimod, in Adult Patients With Eosinophilic Esophagitis: Primary Results from the Phase 2 VOYAGE Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

