Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 2B - Small Intestine / IBD
33 - Symptomatic Improvement Observed Within 2 Days of Etrasimod Induction Therapy: Results from ELEVATE UC 52 and ELEVATE UC 12 Studies in Patients with Ulcerative Colitis
Tuesday, October 24, 2023
9:20 AM - 9:30 AM PT
Location: Ballroom B

Marla C. Dubinsky, MD
Mount Sinai Kravis Children’s Hospital
New York, NY
Presenting Author(s)
Marla C. Dubinsky, MD1, Séverine Vermeire, MD, PhD2, María Chaparro, MD, PhD3, Peter M.. Irving, MD4, Lauren Bartolome, PharmD, MS5, John C.. Woolcott, PhD6, Christopher J.. Rabbat, PhD5, Wenjin Wang, PhD6, Martina Goetsch, MD7, Joana Torres, MD, PhD8, Remo Panaccione, MD9
1Mount Sinai Kravis Children’s Hospital, New York, NY; 2UZ Leuven, Leuven, Vlaams-Brabant, Belgium; 3Hospital Universitario de La Princesa, Instituto de Investigación Sanitaria Princesa (IIS Princesa), Universidad Autónoma de Madrid (UAM), Centro de Investigacíon Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Madrid, Madrid, Spain; 4Guy’s and St. Thomas’ NHS Foundation Trust, London, England, United Kingdom; 5Pfizer Inc., New York, NY; 6Pfizer Inc., Collegeville, PA; 7Pfizer AG, Zurich, Zurich, Switzerland; 8Hospital Beatriz Ângelo, Loures, Lisboa, Portugal; 9University of Calgary, Calgary, AB, Canada
Introduction: The time from treatment initiation to symptom relief, including rectal bleeding (RB) and stool frequency (SF), is key for patients (pts) with ulcerative colitis (UC) and can help guide therapy decisions. Etrasimod is an investigational, oral, once-daily, selective sphingosine 1‑phosphate (S1P)1,4,5 receptor modulator in development for the treatment of moderately to severely active UC. Data from pt e-diaries can help inform on symptoms in pts with UC.
Methods: We present a post hoc analysis of daily e-diary data collected on RB and SF from pts enrolled in the ELEVATE UC 52 (NCT03945188) and ELEVATE UC 12 (NCT03996369) phase 3 clinical trials.1 Data were pooled from the ELEVATE UC 52 and ELEVATE UC 12 studies, which randomized pts with moderately to severely active UC and an inadequate response or loss of response or intolerance to ≥ 1 approved UC therapy 2:1 to receive etrasimod 2 mg once daily or placebo (PBO). Daily Mayo RB and SF subscores (RBS and SFS) and partial modified Mayo score (pMMS; RBS + SFS) were calculated from pt e-diary responses, as well as change from baseline (CFB) during the first 28 days of therapy. Symptomatic responders were pts with ≥ 30% CFB (decrease) in pMMS. Symptomatic remission was defined as pts with RBS=0 plus SFS=0 or 1 (with ≥ 1‑point improvement from baseline). RB and SF remission were defined as pts with RBS=0 and SFS=0, respectively. The Mantel–Haenszel weighted test was used to assess the adjusted risk differences in proportions of responders between treatment groups.
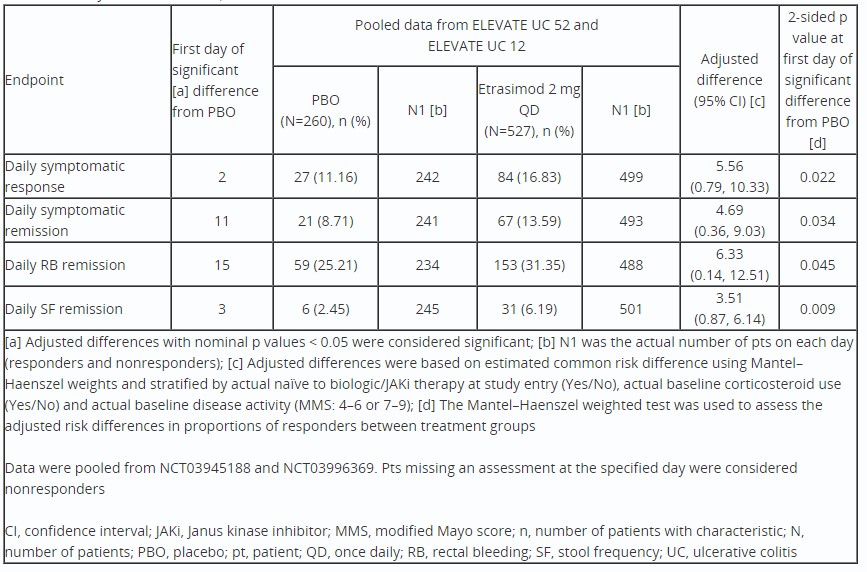
Results: At baseline, pts receiving etrasimod and PBO had a mean (SD) RBS of 1.6 (0.69) and 1.6 (0.68), SFS of 2.4 (0.74) and 2.4 (0.74), and pMMS of 4.0 (1.07) and 4.0 (1.07), respectively. Adjusted differences in symptomatic response and symptomatic remission in pts receiving etrasimod vs PBO became significant from Day 2 (5.56 [0.79, 10.33]) and Day 11 (4.69 [0.36, 9.03]), respectively. Adjusted differences (95% confidence interval) in RB remission and SF remission in pts receiving etrasimod vs PBO reached significance from Day 15 (6.33 [0.14, 12.51]) and Day 3 (3.51 [0.87, 6.14]), respectively (all p < 0.05; Table).
Discussion: In this post hoc analysis of the phase 3 ELEVATE trials, we found significant, early improvements in UC symptoms in pts receiving etrasimod vs PBO beginning within 2 days. These findings indicate a potentially rapid onset of symptomatic effect with etrasimod treatment.
Reference
1. Sandborn WJ et al. Lancet 2023; 401: 1159-1171.
Disclosures:
Marla C. Dubinsky, MD1, Séverine Vermeire, MD, PhD2, María Chaparro, MD, PhD3, Peter M.. Irving, MD4, Lauren Bartolome, PharmD, MS5, John C.. Woolcott, PhD6, Christopher J.. Rabbat, PhD5, Wenjin Wang, PhD6, Martina Goetsch, MD7, Joana Torres, MD, PhD8, Remo Panaccione, MD9, 33, Symptomatic Improvement Observed Within 2 Days of Etrasimod Induction Therapy: Results from ELEVATE UC 52 and ELEVATE UC 12 Studies in Patients with Ulcerative Colitis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Mount Sinai Kravis Children’s Hospital, New York, NY; 2UZ Leuven, Leuven, Vlaams-Brabant, Belgium; 3Hospital Universitario de La Princesa, Instituto de Investigación Sanitaria Princesa (IIS Princesa), Universidad Autónoma de Madrid (UAM), Centro de Investigacíon Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Madrid, Madrid, Spain; 4Guy’s and St. Thomas’ NHS Foundation Trust, London, England, United Kingdom; 5Pfizer Inc., New York, NY; 6Pfizer Inc., Collegeville, PA; 7Pfizer AG, Zurich, Zurich, Switzerland; 8Hospital Beatriz Ângelo, Loures, Lisboa, Portugal; 9University of Calgary, Calgary, AB, Canada
Introduction: The time from treatment initiation to symptom relief, including rectal bleeding (RB) and stool frequency (SF), is key for patients (pts) with ulcerative colitis (UC) and can help guide therapy decisions. Etrasimod is an investigational, oral, once-daily, selective sphingosine 1‑phosphate (S1P)1,4,5 receptor modulator in development for the treatment of moderately to severely active UC. Data from pt e-diaries can help inform on symptoms in pts with UC.
Methods: We present a post hoc analysis of daily e-diary data collected on RB and SF from pts enrolled in the ELEVATE UC 52 (NCT03945188) and ELEVATE UC 12 (NCT03996369) phase 3 clinical trials.1 Data were pooled from the ELEVATE UC 52 and ELEVATE UC 12 studies, which randomized pts with moderately to severely active UC and an inadequate response or loss of response or intolerance to ≥ 1 approved UC therapy 2:1 to receive etrasimod 2 mg once daily or placebo (PBO). Daily Mayo RB and SF subscores (RBS and SFS) and partial modified Mayo score (pMMS; RBS + SFS) were calculated from pt e-diary responses, as well as change from baseline (CFB) during the first 28 days of therapy. Symptomatic responders were pts with ≥ 30% CFB (decrease) in pMMS. Symptomatic remission was defined as pts with RBS=0 plus SFS=0 or 1 (with ≥ 1‑point improvement from baseline). RB and SF remission were defined as pts with RBS=0 and SFS=0, respectively. The Mantel–Haenszel weighted test was used to assess the adjusted risk differences in proportions of responders between treatment groups.
Results: At baseline, pts receiving etrasimod and PBO had a mean (SD) RBS of 1.6 (0.69) and 1.6 (0.68), SFS of 2.4 (0.74) and 2.4 (0.74), and pMMS of 4.0 (1.07) and 4.0 (1.07), respectively. Adjusted differences in symptomatic response and symptomatic remission in pts receiving etrasimod vs PBO became significant from Day 2 (5.56 [0.79, 10.33]) and Day 11 (4.69 [0.36, 9.03]), respectively. Adjusted differences (95% confidence interval) in RB remission and SF remission in pts receiving etrasimod vs PBO reached significance from Day 15 (6.33 [0.14, 12.51]) and Day 3 (3.51 [0.87, 6.14]), respectively (all p < 0.05; Table).
Discussion: In this post hoc analysis of the phase 3 ELEVATE trials, we found significant, early improvements in UC symptoms in pts receiving etrasimod vs PBO beginning within 2 days. These findings indicate a potentially rapid onset of symptomatic effect with etrasimod treatment.
Reference
1. Sandborn WJ et al. Lancet 2023; 401: 1159-1171.

Table: Table. Responses in Pts Receiving Etrasimod Compared with PBO on the First Day of a Significant Adjusted Difference in Daily Symptomatic Response, Symptomatic Remission, RB Remission and SF Remission.
Disclosures:
Marla Dubinsky: AbbVie – Consultant, Grant/Research Support. Arena Pharmaceuticals – Consultant. Astra Zeneca – Consultant. Celgene – Consultant. Genentech Inc. – Consultant. Gilead Sciences – Consultant. Janssen – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant, Grant/Research Support. Prometheus Biosciences – Consultant, Grant/Research Support. Prometheus Labs – Consultant, Grant/Research Support. Takeda – Consultant, Licensing fees. Thabor – Consultant. Trellus Health – Stock-publicly held company(excluding mutual/index funds). UCB Pharma – Consultant.
Séverine Vermeire: AbbVie – Consultant, Grant/Research Support. AbolerIS Pharma – Grant/Research Support. AgomAb – Grant/Research Support. Alimentiv – Consultant. Arena Pharmaceuticals – Consultant. AstraZeneca – Consultant. Avaxia Biologics – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. CVasThera – Consultant, Speakers Bureau. Cytoki Pharma – Consultant, Speakers Bureau. Dr. Falk Pharma – Consultant, Speakers Bureau. Eli Lilly – Consultant, Speakers Bureau. Ferring Pharmaceuticals – Consultant, Speakers Bureau. Galapagos – Consultant, Grant/Research Support, Speakers Bureau. Genentech/Roche – Consultant, Speakers Bureau. Gilead – Consultant, Speakers Bureau. GSK – Consultant, Speakers Bureau. Hospira – Consultant, Speakers Bureau. IMIDomics – Consultant, Speakers Bureau. Janssen Pharmaceuticals – Consultant, Speakers Bureau. Johnson & Johnson – Consultant, Grant/Research Support, Speakers Bureau. Materia Prima – Consultant, Speakers Bureau. MiroBio – Consultant, Speakers Bureau. Morphic – Consultant, Speakers Bureau. MRM Health – Consultant, Speakers Bureau. MSD – Consultant, Speakers Bureau. Mundipharma – Consultant, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. ProDigest – Consultant, Speakers Bureau. Progenity – Consultant, Speakers Bureau. Prometheus – Consultant, Speakers Bureau. Robarts Clinical Trials – Consultant, Speakers Bureau. Second Genome – Consultant, Speakers Bureau. Shire – Consultant, Speakers Bureau. Surrozen – Consultant, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau. Theravance Biopharma – Consultant, Speakers Bureau. Tillotts Pharma – Consultant, Speakers Bureau. Zealand Pharma – Consultant, Speakers Bureau.
María Chaparro: Gilead – Consultant. Janssen – Consultant. Lilly – Consultant. Pfizer Inc – Consultant. Takeda – Consultant. Tillots – Consultant.
Peter Irving: AbbVie – Advisory Committee/Board Member, lecture fees. Arena – Advisory Committee/Board Member. Boehringer-Ingelheim – Advisory Committee/Board Member. Bristol Myers Squibb – Advisory Committee/Board Member, lecture fees. Celgene – Advisory Committee/Board Member, lecture fees. Celltrion – Advisory Committee/Board Member, Grant/Research Support, lecture fees. Eli Lilly – Advisory Committee/Board Member, lecture fees. Falk Pharma – lecture fees. Ferring – lecture fees. Galapagos – Grant/Research Support, lecture fees. Genetech – Advisory Committee/Board Member. Gilead – Advisory Committee/Board Member, lecture fees. Hospira – Advisory Committee/Board Member. Janssen – Advisory Committee/Board Member, lecture fees. MSD – Advisory Committee/Board Member, Grant/Research Support, lecture fees. Pfizer – Advisory Committee/Board Member, Grant/Research Support, lecture fees. Pharmacosmos – Advisory Committee/Board Member. Prometheus – Advisory Committee/Board Member. Roche – Advisory Committee/Board Member. Samsung Bioepis – Advisory Committee/Board Member. Sandoz – Advisory Committee/Board Member, lecture fees. Sapphire Medical – lecture fees. Shire – lecture fees. Takeda – Advisory Committee/Board Member, Grant/Research Support, lecture fees. Tillots – lecture fees. Topivert – Advisory Committee/Board Member. VH2 – Advisory Committee/Board Member. Vifor Pharma – Advisory Committee/Board Member. Warner Chilcott – Advisory Committee/Board Member, lecture fees.
Lauren Bartolome: Pfizer Inc – Employee, Stock Options.
John Woolcott: Pfizer Inc – Employee, Stock Options.
Christopher Rabbat: Pfizer Inc – Employee, Stock Options.
Wenjin Wang: Pfizer Inc – Employee, Stock Options.
Martina Goetsch: Pfizer AG – Employee. Pfizer Inc – Stock-publicly held company(excluding mutual/index funds).
Joana Torres: AbbVie – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Bristol-Myers Squibb – Advisory Committee/Board Member. Janssen – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Pfizer Inc – Advisory Committee/Board Member, Speakers Bureau.
Remo Panaccione: Abbivax – Consultant. Abbott – Consultant. AbbVie – Consultant. Alimentiv – Consultant. Amgen – Consultant. Arena – Consultant. AstraZeneca – Consultant. Biogen – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. Celltrion – Consultant. Cosmos Technology – Consultant. Eisai – Consultant. Elan – Consultant. Eli Lilly – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Galapagos – Consultant. Genentech – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. JAMP Bio – Consultant. Janssen – Consultant. Merck – Consultant. Mylan – Consultant. Novartis – Consultant. Oppilan Pharma – Consultant. Organon – Consultant. Pandion Pharma – Consultant. Pendopharm – Consultant. Pfizer Inc – Consultant. Progenity – Consultant. Prometheus – Consultant. Protagonist Therapeutics – Consultant. Roche – Consultant. Sandoz – Consultant. Satisfai Health – Consultant. Shire – Consultant. Sublimity Therapeutics – Consultant. Takeda Pharmaceuticals – Consultant. Theravance Biopharma – Consultant. Trellus – Consultant. UCB – Consultant. Ventyx – Consultant. Viatris – Consultant.
Marla C. Dubinsky, MD1, Séverine Vermeire, MD, PhD2, María Chaparro, MD, PhD3, Peter M.. Irving, MD4, Lauren Bartolome, PharmD, MS5, John C.. Woolcott, PhD6, Christopher J.. Rabbat, PhD5, Wenjin Wang, PhD6, Martina Goetsch, MD7, Joana Torres, MD, PhD8, Remo Panaccione, MD9, 33, Symptomatic Improvement Observed Within 2 Days of Etrasimod Induction Therapy: Results from ELEVATE UC 52 and ELEVATE UC 12 Studies in Patients with Ulcerative Colitis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

