Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 2B - Small Intestine / IBD
32 - Pharmacokinetics, Exposure-Response Relationships, and Immunogenicity in Crohn’s Disease Patients with Ustekinumab Secondary Loss of Response Following Ustekinumab IV Re-Induction: Week 16 Results from the POWER Study
Tuesday, October 24, 2023
9:10 AM - 9:20 AM PT
Location: Ballroom B
.jpg)
Brian G. Feagan, MD
Western University
London, ON, Canada
Presenting Author(s)
Brian G. Feagan, MD1, Scott D. Lee, MD2, C. Janneke van der Woude, MD, PhD3, Ignacio Marín-Jiménez, MD4, Douglas C.. Wolf, MD5, Elisabeth Schnoy, MD, PhD6, Bruce Salzberg, MD7, Christopher Busse, 8, Maciej Nazar, 9, Wayne Langholff, 10, Christopher Gasink, 8, Thomas Baker, 10, Bridget Godwin, MD8, Omoniyi Adedokun, 10, Stefan Schreiber, MD11
1Western University, London, ON, Canada; 2University of Washington, Seattle, WA; 3Erasmus University Medical Center, Rotterdam, Drenthe, Netherlands; 4Hospital Universitario Gregorio Marañón, IiSGM, Rotterdam, Zuid-Holland, Netherlands; 5Atlanta Gastroenterology Associates, Atlanta, GA; 6University Hospital of Augsburg, Augsburg, Bayern, Germany; 7Atlanta Gastroenterology Specialists PC, IBD Center of Atlanta, Atlanta, GA; 8Janssen Scientific Affairs, LLC, Horsham, PA; 9Janssen-Cilag Polska Sp. z o.o, Warsaw, Mazowieckie, Poland; 10Janssen Research & Development, LLC, Spring House, PA; 11University Hospital Schleswig-Holstein, Kiel, Schleswig-Holstein, Germany
Introduction: The POWER study evaluated efficacy and safety of a single intravenous (IV) re-induction ustekinumab (UST) dose vs continued UST subcutaneous (SC) treatment in Crohn’s disease (CD) patients (pts) with secondary loss of response (LoR) to standard UST maintenance therapy. Here, we present Week (W)16 pharmacokinetics, exposure–response relationships, and immunogenicity analysis.
Methods: Adult pts with moderately–severely active CD who initially responded to UST IV induction therapy per label and later experienced LoR were included. LoR was defined as CD Activity Index (CDAI) score ≥220–≤450, plus elevated C-reactive protein ( >3mg/L)/fecal calprotectin ( >250mg/kg), or endoscopy performed ≤3 months before baseline (W0) with active CD. At W0, randomized pts received ~6mg/kg IV UST, or SC UST 90mg, followed by SC UST 90mg dosing at W8/16. Clinical assessments occurred at W0/8/16. Serum samples evaluated UST concentration (conc) and anti-drug antibodies (ADAs) at W0/8/16 using validated assays. Clinical response (CRes) and endoscopic remission (ERem) were evaluated based on CDAI and centrally read endoscopy, respectively, at W0/8/16. Efficacy outcomes were analyzed by W0 trough conc groups (undetectable, 0.17–< 0.8, 0.8–< 1.3, and ≥1.3µg/mL) and conc quartile groups (QGs) at W16 using a proprietary immunoassay.
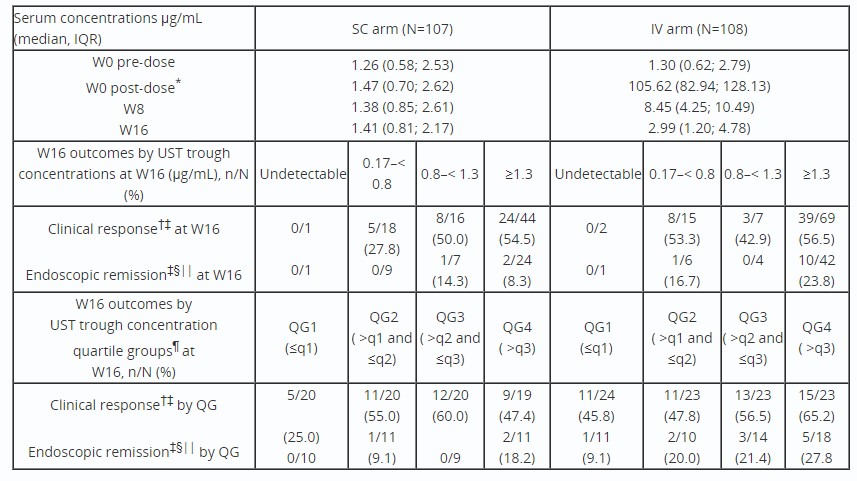
Results: Serum UST conc data were obtained from 215 pts (SC, n=107; IV, n=108) with ≥1 blood sample collected. Similar at W0, median serum UST concs at W0 (1-hour post-dose)/8/16 were higher in the IV vs SC arm (Table). Based on W16 trough concs, 63.9% (69/108) of pts were in the ≥1.3µg/mL group in the IV arm, vs 41.1% (44/107) in the SC arm. The proportion of pts with W16 CRes in the ≥1.3µg/mL group was similar between arms; more pts were in W16 ERem in the IV vs SC arm. By W16 quartile analysis, CRes was numerically higher in the IV vs SC arm in QG1 and 4. More IV arm pts achieved ERem in QG4 vs QG1; a small number of pts in SC arm achieved ERem independent of QGs. Incidence of UST ADAs through W16 was low (SC, n=2; IV, n=0; 0.93% overall).
Discussion: Although, IV re-induction resulted in higher serum concs and proportions of pts with high (≥1.3µg/mL) trough conc at W16; there did not appear to be an exposure-response relationship for CRes. However, quartile analysis showed a relationship between increased exposure in the IV arm and differences in ERem. This observation is consistent with the notion that pts with secondary LoR may benefit from single-dose re-induction.
Disclosures:
Brian G. Feagan, MD1, Scott D. Lee, MD2, C. Janneke van der Woude, MD, PhD3, Ignacio Marín-Jiménez, MD4, Douglas C.. Wolf, MD5, Elisabeth Schnoy, MD, PhD6, Bruce Salzberg, MD7, Christopher Busse, 8, Maciej Nazar, 9, Wayne Langholff, 10, Christopher Gasink, 8, Thomas Baker, 10, Bridget Godwin, MD8, Omoniyi Adedokun, 10, Stefan Schreiber, MD11, 32, Pharmacokinetics, Exposure-Response Relationships, and Immunogenicity in Crohn’s Disease Patients with Ustekinumab Secondary Loss of Response Following Ustekinumab IV Re-Induction: Week 16 Results from the POWER Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Western University, London, ON, Canada; 2University of Washington, Seattle, WA; 3Erasmus University Medical Center, Rotterdam, Drenthe, Netherlands; 4Hospital Universitario Gregorio Marañón, IiSGM, Rotterdam, Zuid-Holland, Netherlands; 5Atlanta Gastroenterology Associates, Atlanta, GA; 6University Hospital of Augsburg, Augsburg, Bayern, Germany; 7Atlanta Gastroenterology Specialists PC, IBD Center of Atlanta, Atlanta, GA; 8Janssen Scientific Affairs, LLC, Horsham, PA; 9Janssen-Cilag Polska Sp. z o.o, Warsaw, Mazowieckie, Poland; 10Janssen Research & Development, LLC, Spring House, PA; 11University Hospital Schleswig-Holstein, Kiel, Schleswig-Holstein, Germany
Introduction: The POWER study evaluated efficacy and safety of a single intravenous (IV) re-induction ustekinumab (UST) dose vs continued UST subcutaneous (SC) treatment in Crohn’s disease (CD) patients (pts) with secondary loss of response (LoR) to standard UST maintenance therapy. Here, we present Week (W)16 pharmacokinetics, exposure–response relationships, and immunogenicity analysis.
Methods: Adult pts with moderately–severely active CD who initially responded to UST IV induction therapy per label and later experienced LoR were included. LoR was defined as CD Activity Index (CDAI) score ≥220–≤450, plus elevated C-reactive protein ( >3mg/L)/fecal calprotectin ( >250mg/kg), or endoscopy performed ≤3 months before baseline (W0) with active CD. At W0, randomized pts received ~6mg/kg IV UST, or SC UST 90mg, followed by SC UST 90mg dosing at W8/16. Clinical assessments occurred at W0/8/16. Serum samples evaluated UST concentration (conc) and anti-drug antibodies (ADAs) at W0/8/16 using validated assays. Clinical response (CRes) and endoscopic remission (ERem) were evaluated based on CDAI and centrally read endoscopy, respectively, at W0/8/16. Efficacy outcomes were analyzed by W0 trough conc groups (undetectable, 0.17–< 0.8, 0.8–< 1.3, and ≥1.3µg/mL) and conc quartile groups (QGs) at W16 using a proprietary immunoassay.
Results: Serum UST conc data were obtained from 215 pts (SC, n=107; IV, n=108) with ≥1 blood sample collected. Similar at W0, median serum UST concs at W0 (1-hour post-dose)/8/16 were higher in the IV vs SC arm (Table). Based on W16 trough concs, 63.9% (69/108) of pts were in the ≥1.3µg/mL group in the IV arm, vs 41.1% (44/107) in the SC arm. The proportion of pts with W16 CRes in the ≥1.3µg/mL group was similar between arms; more pts were in W16 ERem in the IV vs SC arm. By W16 quartile analysis, CRes was numerically higher in the IV vs SC arm in QG1 and 4. More IV arm pts achieved ERem in QG4 vs QG1; a small number of pts in SC arm achieved ERem independent of QGs. Incidence of UST ADAs through W16 was low (SC, n=2; IV, n=0; 0.93% overall).
Discussion: Although, IV re-induction resulted in higher serum concs and proportions of pts with high (≥1.3µg/mL) trough conc at W16; there did not appear to be an exposure-response relationship for CRes. However, quartile analysis showed a relationship between increased exposure in the IV arm and differences in ERem. This observation is consistent with the notion that pts with secondary LoR may benefit from single-dose re-induction.

Table: Table: Summary of serum UST concentrations and the proportion of patients in clinical response and endoscopic remission at W16 by UST trough concentrations and trough concentration quartile groups at W16 (full analysis set).
*The post-dose sample was collected within 24 hours of UST administration.
†Clinical response is defined by CDAI <150 or decrease of ≥100 points from W0.
‡Patients who had insufficient data to calculate CDAI or SES-CD, a prohibited CD-related surgery, prohibited concomitant medication changes, or discontinued study agent due to lack of efficacy or due to an adverse event indicated to be of worsening CD prior to the designated analysis timepoint are considered not to be in clinical response or endoscopic remission.
§Endoscopic remission is defined as an SES-CD score ≤3 or SES-CD=0 for patients who enter the study with an SES-CD=3.
||Endoscopy was an optional procedure in the study; only 58 (SC arm) and 59 (IV arm) patients were included in the endoscopic analysis.
¶Quartiles are based on patients in each treatment group: SC arm, q1=0.81 µg/mL, q2=1.41 µg/mL, q3=2.17 µg/mL; IV arm, q1=1.20 µg/mL, q2=2.99 µg/mL, q3=4.78 µg/mL.
CD, Crohn’s Disease; CDAI, Crohn’s Disease Activity Index; IQR, interquartile range; IV, intravenous; q, quartile; QG, quartile group; SC, subcutaneous; SES-CD; Simple Endoscopic Score in Crohn’s Disease; UST, ustekinumab; W, week.
*The post-dose sample was collected within 24 hours of UST administration.
†Clinical response is defined by CDAI <150 or decrease of ≥100 points from W0.
‡Patients who had insufficient data to calculate CDAI or SES-CD, a prohibited CD-related surgery, prohibited concomitant medication changes, or discontinued study agent due to lack of efficacy or due to an adverse event indicated to be of worsening CD prior to the designated analysis timepoint are considered not to be in clinical response or endoscopic remission.
§Endoscopic remission is defined as an SES-CD score ≤3 or SES-CD=0 for patients who enter the study with an SES-CD=3.
||Endoscopy was an optional procedure in the study; only 58 (SC arm) and 59 (IV arm) patients were included in the endoscopic analysis.
¶Quartiles are based on patients in each treatment group: SC arm, q1=0.81 µg/mL, q2=1.41 µg/mL, q3=2.17 µg/mL; IV arm, q1=1.20 µg/mL, q2=2.99 µg/mL, q3=4.78 µg/mL.
CD, Crohn’s Disease; CDAI, Crohn’s Disease Activity Index; IQR, interquartile range; IV, intravenous; q, quartile; QG, quartile group; SC, subcutaneous; SES-CD; Simple Endoscopic Score in Crohn’s Disease; UST, ustekinumab; W, week.
Disclosures:
Brian Feagan: AbbVie – Advisory Committee/Board Member, Consultant, Speakers Bureau. AbolerIS – Consultant. AgomAB Therapeutics – Consultant. Allianthera – Consultant. Amgen – Advisory Committee/Board Member, Consultant. AnaptysBio – Consultant. Applied Molecular Transport Inc. – Consultant. Arena Pharma – Consultant. Avir – Consultant. Azora Therapeutics – Consultant. Baxter – Consultant. BioJamp – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Boston Pharma – Consultant. Bristol Myers Squibb – Consultant. Celgene/BMS – Advisory Committee/Board Member, Consultant. Connect BioPharma – Consultant. Cytoki – Consultant. Disc Medicine – Consultant. Duality – Consultant. EcoR1 – Advisory Committee/Board Member, Consultant. Eli Lilly – Consultant. Equillium – Consultant. Ermium – Consultant. Everest Clinical Research Corp. – Consultant. Ferring Pharmaceuticals – Consultant. First Wave – Consultant. Galapagos – Consultant. Galen Atlantica – Consultant. Genentech/Roche – Advisory Committee/Board Member, Consultant. Gilead – Consultant. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. Glenmark – Consultant. Gossamer Pharma – Consultant, stock shareholder. Hoffmann-LaRoche – Consultant. Hot Spot Therapeutics – Consultant. Imhotex – Consultant. ImmunExt – Consultant. Immunic Therapeutics – Consultant. Index Pharma – Advisory Committee/Board Member, Consultant. Intact Therapeutics – Consultant. JAKAcademy – Consultant. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Japan Tobacco Inc. – Consultant. Kaleido Biosciences – Consultant. L.E.K. Consulting – Consultant. Landos Biopharma – Consultant. Leadiant – Consultant. LifeSci Capital – Consultant. Lument AB – Consultant. Millennium – Consultant. MiroBio – Consultant. Morphic Therapeutics – Advisory Committee/Board Member, Consultant. Mylan – Consultant. Novartis – Advisory Committee/Board Member. OM Pharma – Consultant. Origo BioPharma – Advisory Committee/Board Member, Consultant. Orphagen – Consultant. Otsuka – Consultant. Pandion Therapeutics – Consultant. Pfizer – Advisory Committee/Board Member, Consultant. Play to Know AG – Consultant. Progenity – Advisory Committee/Board Member, Consultant. Prometheus Therapeutics and Diagnostics – Advisory Committee/Board Member, Consultant. Protagonist – Consultant. PTM Therapeutics – Consultant. Q32 Bio – Consultant. Rebiotix – Consultant. RedHill Biopharma – Consultant. Redx – Consultant. Roche – Consultant. Sandoz – Consultant. Sanofi – Consultant. Seres Therapeutics – Consultant. Silverback Therapeutics – Consultant. Surrozen Inc. – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Speakers Bureau. Teva – Advisory Committee/Board Member, Consultant. Thelium – Consultant. Theravance – Consultant. Tigenix – Consultant. Tillotts Pharma – Advisory Committee/Board Member, Consultant. UCB Pharma – Consultant. VHSquared Ltd. – Consultant. Viatris – Consultant. Western University, Alimentiv Inc – Employee. Ysios – Consultant. Ysopia – Consultant. Zealand Pharma – Consultant.
Scott Lee: AbbVie – Grant/Research Support. AbGenomics – Grant/Research Support. Applied Molecular Transport, Arena, Boehringer Ingelheim – Consultant. Arena – Grant/Research Support. Bridge Biotherapeutics – Consultant. Bristol-Myers Squibb – Consultant. Celgene – Consultant. Celgene – Grant/Research Support. Celltrion Healthcare – Consultant. Celltrion Healthcare – Grant/Research Support. Cornerstones Health – Consultant. Eli Lilly and Company – Consultant. Janssen – Consultant. Janssen – Grant/Research Support. KCRN Research – Consultant. Salix – Grant/Research Support. Samsung Bioepis and UCB – Consultant. Takeda – Grant/Research Support. UCB – Grant/Research Support.
C. Janneke van der Woude: AbbVie – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Celltrion – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Dr. Falk Pharma Benelux – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Falk Ben-elux – Grant/Research Support. Ferring – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Janssen – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Pfizer – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Takeda – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau.
Ignacio Marín-Jiménez: Abbvie – Advisory Committee/Board Member, Consultant, Grant/Research Support. Chiesi – Advisory Committee/Board Member, Consultant, Grant/Research Support. Faes Farma – Advisory Committee/Board Member, Consultant, Grant/Research Support. FalkPharma – Advisory Committee/Board Member, Consultant, Grant/Research Support. Ferring – Advisory Committee/Board Member, Consultant, Grant/Research Support. Gebro Pharma – Advisory Committee/Board Member, Consultant, Grant/Research Support. Hospira – Advisory Committee/Board Member, Consultant, Grant/Research Support. Janssen – Advisory Committee/Board Member, Consultant, Grant/Research Support. MSD – Advisory Committee/Board Member, Consultant, Grant/Research Support. Otsuka Pharmaceutical – Advisory Committee/Board Member, Consultant, Grant/Research Support. Pfizer – Advisory Committee/Board Member, Consultant, Grant/Research Support. Shire – Advisory Committee/Board Member, Consultant, Grant/Research Support. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support. Tillots – Advisory Committee/Board Member, Consultant, Grant/Research Support. UCB Pharma – Advisory Committee/Board Member, Consultant, Grant/Research Support.
Douglas Wolf: AbbVie – Advisory Committee/Board Member, Consultant, Speakers Bureau. Arena – Advisory Committee/Board Member, Consultant, Speakers Bureau. Celgene/Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speakers Bureau. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Pfizer – Advisory Committee/Board Member, Consultant, Speakers Bureau. Takeda – Advisory Committee/Board Member, Consultant, Speakers Bureau.
Elisabeth Schnoy: AbbVie – personal fees. Bristol Myers Squibb – personal fees. Celltrion Healthcare – personal fees. Dr. Falk Pharma – personal fees. Galapagos/Gilead – personal fees. I-Mab – personal fees. Janssen – personal fees. Lilly – personal fees. Pfizer – personal fees. Pharmacosmos – personal fees. Takeda – personal fees. Tillotts Pharma – personal fees.
Bruce Salzberg: AbbVie – honoraria as a speaker. Bristol-Myers Squibb – honoraria as a speaker. Johnson and Johnson – Consultant. Johnson and Johnson – honoraria as a speaker.
Christopher Busse: Janssen Research & Development, LLC – Employee. Johnson & Johnson – Stock-publicly held company(excluding mutual/index funds).
Maciej Nazar: Janssen-Cilag Polska Sp. z o.o. – Employee, Stock-publicly held company(excluding mutual/index funds).
Wayne Langholff: Janssen Research & Development, LLC – Employee. Johnson & Johnson – Stock-publicly held company(excluding mutual/index funds).
Christopher Gasink: Janssen Research & Development, LLC – Employee. Johnson & Johnson – Stock-publicly held company(excluding mutual/index funds).
Thomas Baker: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Bridget Godwin: Janssen Research & Development, LLC – Employee. Johnson & Johnson – Stock-publicly held company(excluding mutual/index funds).
Omoniyi Adedokun: Janssen Research & Development, LLC – Employee. Johnson & Johnson – Stock-publicly held company(excluding mutual/index funds).
Stefan Schreiber: AbbVie – Personal fees. Amgen – Personal fees. Arena Pharmaceuticals – Personal fees. Biogen – Personal fees. Bristol Myers Squibb – Personal fees. Celgene – Personal fees. Celltrion Healthcare – Personal fees. Dr. Falk Pharma – Personal fees. Eli Lilly – personal fees. Ferring Pharmaceuticals – personal fees. Fresenius Kabi – Personal fees. Galapagos – Personal fees. Gilead – Personal fees. Hikma Pharmaceuticals – Personal fees. I-Mab – Personal fees. Janssen Pharmaceuticals – Personal fees. Morphic – Personal fees. MSD – Personal fees. Mylan – Personal fees. Pfizer – Personal fees. Protagonist – Personal fees. ProventionBio – Personal fees. Sandoz/Hexal – personal fees. Takeda – Personal fees. Theravance Biopharma – Personal fees. UCB – personal fees.
Brian G. Feagan, MD1, Scott D. Lee, MD2, C. Janneke van der Woude, MD, PhD3, Ignacio Marín-Jiménez, MD4, Douglas C.. Wolf, MD5, Elisabeth Schnoy, MD, PhD6, Bruce Salzberg, MD7, Christopher Busse, 8, Maciej Nazar, 9, Wayne Langholff, 10, Christopher Gasink, 8, Thomas Baker, 10, Bridget Godwin, MD8, Omoniyi Adedokun, 10, Stefan Schreiber, MD11, 32, Pharmacokinetics, Exposure-Response Relationships, and Immunogenicity in Crohn’s Disease Patients with Ustekinumab Secondary Loss of Response Following Ustekinumab IV Re-Induction: Week 16 Results from the POWER Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

