Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 3B - General Endoscopy / Practice Management / Colorectal Cancer Prevention
54 - Next-Generation Multitarget Stool DNA Test for Colorectal Cancer Screening: A Prospective Clinical Validation Study (BLUE-C) (Late-Breaking Abstract)
Tuesday, October 24, 2023
3:35 PM - 3:45 PM PT
Location: Ballroom B

Thomas F. Imperiale, MD
Professor of Medicine
Indiana University Medical Center
Indianapolis, IN
Late Breaking Abstract Presenter(s)
Thomas F. Imperiale, MD1, Kyle Porter, MAS2, Julia Zella, PhD2, Zubin D. Gagrat, BS2, Marilyn C. Olson, PhD2, Sandi Statz, MS2, Jorge Garces, PhD2, Philip Lavin, PhD3, Humberto Aguilar, MD4, Don Brinberg, MD5, Charles Berkelhammer, MD6, John B. Kisiel, MD7, Paul J. Limburg, MD2; 1Indiana University School of Medicine, Indianapolis, IN; 2Exact Sciences, Madison, WI; 3Boston Biostatistics Research Foundation, Framingham, MA; 4Louisiana Research Center, Shreveport, LA; 5Great Lakes Gastroenterology Research, Mentor, OH; 6Southwest Gastroenterology, Oak Lawn, IL; 7Mayo Clinic, Rochester, MN.
Introduction: The multitarget stool DNA (mt-sDNA) test is a non-invasive, average-risk colorectal cancer (CRC) screening test. To enhance clinical performance (particularly specificity), we developed a next-generation (next-gen) mt-sDNA test that combines 3 novel methylated DNA markers and fecal hemoglobin. We report results of a prospective, cross-sectional study (BLUE-C; NCT04144738) to determine next-gen mt-sDNA test performance.
Methods: Eligible adults (>40 years) scheduled for screening colonoscopy were consented, enrolled at 186 sites in the United States, and provided a stool sample for the next-gen mt-sDNA test and comparator fecal immunochemical test (FIT; OC-Auto® Micro 80, Polymedco, Inc.) prior to colonoscopy preparation. Colonoscopy findings were categorized as CRC, advanced precancerous lesions (APL; lesions with high grade dysplasia, ≥25% villous morphology, or ≥10 mm in size), no advanced neoplasia (absence of CRC or APL), and non-neoplastic findings or negative colonoscopy. Next-gen mt-sDNA test markers, algorithm, and cut-off were locked prior to sample processing by a central laboratory blinded to clinical study data. Primary outcomes were CRC sensitivity and specificity for absence of advanced neoplasia. Secondary outcomes were APL sensitivity, specificity for non-neoplastic findings or negative colonoscopy, and comparison of CRC and APL sensitivity for the next-gen mt-sDNA test versus FIT.
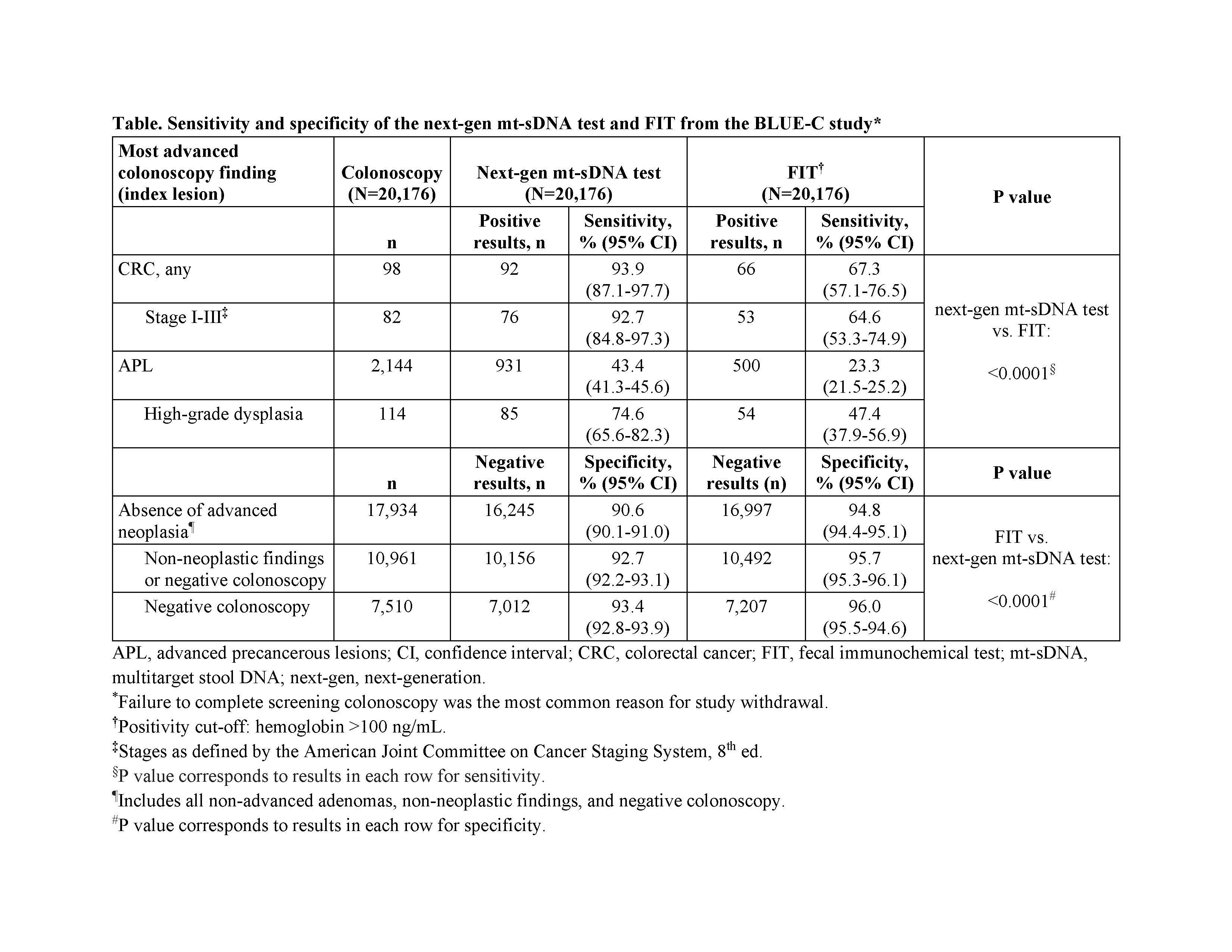
Results: Among 26,758 participants enrolled from 11/15/2019 to 1/5/2023, 20,176 (75.4%) were evaluable (mean age 63.0 years; 53.2% female; 60.1% non-Hispanic White). Colonoscopy findings included 98 CRC, 2,144 APL, and 17,934 with no advanced neoplasia (6,973 with non-advanced adenomas; 10,961 with non-neoplastic findings or negative colonoscopy). Next-gen mt-sDNA test specificity for absence of advanced neoplasia was 90.6% (95% CI, 90.1-91.0; Table). CRC sensitivity was 93.9% (95% CI, 87.1-97.7). APL sensitivity was 43.4% (95% CI, 41.3-45.6); sensitivity for APL with high-grade dysplasia was 74.6% (95% CI, 65.6-82.3). Specificity for non-neoplastic findings or negative colonoscopy was 92.7% (95% CI, 92.2-93.1). Next-gen mt-sDNA test CRC and APL sensitivities were significantly higher than FIT (P<0.0001; Table).
Discussion: In this prospective clinical validation study, the next-gen mt-sDNA test demonstrated high specificity and high CRC and APL sensitivity, outperforming FIT for CRC and APL sensitivity but not specificity.
Disclosures:
Thomas F. Imperiale, MD1, Kyle Porter, MAS2, Julia Zella, PhD2, Zubin D. Gagrat, BS2, Marilyn C. Olson, PhD2, Sandi Statz, MS2, Jorge Garces, PhD2, Philip Lavin, PhD3, Humberto Aguilar, MD4, Don Brinberg, MD5, Charles Berkelhammer, MD6, John B. Kisiel, MD7, Paul J. Limburg, MD2; 1Indiana University School of Medicine, Indianapolis, IN; 2Exact Sciences, Madison, WI; 3Boston Biostatistics Research Foundation, Framingham, MA; 4Louisiana Research Center, Shreveport, LA; 5Great Lakes Gastroenterology Research, Mentor, OH; 6Southwest Gastroenterology, Oak Lawn, IL; 7Mayo Clinic, Rochester, MN. 54 - Next-Generation Multitarget Stool DNA Test for Colorectal Cancer Screening: A Prospective Clinical Validation Study (BLUE-C) (Late-Breaking Abstract), ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
Introduction: The multitarget stool DNA (mt-sDNA) test is a non-invasive, average-risk colorectal cancer (CRC) screening test. To enhance clinical performance (particularly specificity), we developed a next-generation (next-gen) mt-sDNA test that combines 3 novel methylated DNA markers and fecal hemoglobin. We report results of a prospective, cross-sectional study (BLUE-C; NCT04144738) to determine next-gen mt-sDNA test performance.
Methods: Eligible adults (>40 years) scheduled for screening colonoscopy were consented, enrolled at 186 sites in the United States, and provided a stool sample for the next-gen mt-sDNA test and comparator fecal immunochemical test (FIT; OC-Auto® Micro 80, Polymedco, Inc.) prior to colonoscopy preparation. Colonoscopy findings were categorized as CRC, advanced precancerous lesions (APL; lesions with high grade dysplasia, ≥25% villous morphology, or ≥10 mm in size), no advanced neoplasia (absence of CRC or APL), and non-neoplastic findings or negative colonoscopy. Next-gen mt-sDNA test markers, algorithm, and cut-off were locked prior to sample processing by a central laboratory blinded to clinical study data. Primary outcomes were CRC sensitivity and specificity for absence of advanced neoplasia. Secondary outcomes were APL sensitivity, specificity for non-neoplastic findings or negative colonoscopy, and comparison of CRC and APL sensitivity for the next-gen mt-sDNA test versus FIT.
Results: Among 26,758 participants enrolled from 11/15/2019 to 1/5/2023, 20,176 (75.4%) were evaluable (mean age 63.0 years; 53.2% female; 60.1% non-Hispanic White). Colonoscopy findings included 98 CRC, 2,144 APL, and 17,934 with no advanced neoplasia (6,973 with non-advanced adenomas; 10,961 with non-neoplastic findings or negative colonoscopy). Next-gen mt-sDNA test specificity for absence of advanced neoplasia was 90.6% (95% CI, 90.1-91.0; Table). CRC sensitivity was 93.9% (95% CI, 87.1-97.7). APL sensitivity was 43.4% (95% CI, 41.3-45.6); sensitivity for APL with high-grade dysplasia was 74.6% (95% CI, 65.6-82.3). Specificity for non-neoplastic findings or negative colonoscopy was 92.7% (95% CI, 92.2-93.1). Next-gen mt-sDNA test CRC and APL sensitivities were significantly higher than FIT (P<0.0001; Table).
Discussion: In this prospective clinical validation study, the next-gen mt-sDNA test demonstrated high specificity and high CRC and APL sensitivity, outperforming FIT for CRC and APL sensitivity but not specificity.

Disclosures:
Thomas Imperiale - Grant/Research Support, Exact Sciences
Kyle Porter - Employee, Exact Sciences; Stock/Stock Options, Exact Sciences
Julia Zella - Employee, Exact Sciences; Stock/Stock Options, Exact Sciences
Zubin D. Gagrat - Employee, Exact Sciences; Stock/Stock Options, Exact Sciences; Intellectual Property/Patents, Exact Sciences
Marilyn C. Olson - Employee, Exact Sciences; Stock/Stock Options, Exact Sciences
Sandi Statz - Employee (former), Exact Sciences; Stock, Exact Sciences
Philip Lavin - Grant/Research Support, Exact Sciences (paid to Boston Biostatistics Research Foundation)
Jorge Garces - Employee, Exact Sciences; Stock/Stock Options, Exact Sciences
Humberto Aguilar indicated no relevant financial relationships.
Don Brinberg - Grant/Research Support, Exact Sciences (paid to Great Lakes Gastroenterology Research)
Charles Berkelhammer indicated no relevant financial relationships.
John B. Kisiel - Grant/Research Support (paid to Mayo Clinic), Exact Sciences; Intellectual Property/Patents, Exact Sciences; Royalties (paid to Mayo Clinic), Exact Sciences
Paul J. Limburg - Employee, Exact Sciences; Stock/Stock Options, Exact Sciences.
Thomas F. Imperiale, MD1, Kyle Porter, MAS2, Julia Zella, PhD2, Zubin D. Gagrat, BS2, Marilyn C. Olson, PhD2, Sandi Statz, MS2, Jorge Garces, PhD2, Philip Lavin, PhD3, Humberto Aguilar, MD4, Don Brinberg, MD5, Charles Berkelhammer, MD6, John B. Kisiel, MD7, Paul J. Limburg, MD2; 1Indiana University School of Medicine, Indianapolis, IN; 2Exact Sciences, Madison, WI; 3Boston Biostatistics Research Foundation, Framingham, MA; 4Louisiana Research Center, Shreveport, LA; 5Great Lakes Gastroenterology Research, Mentor, OH; 6Southwest Gastroenterology, Oak Lawn, IL; 7Mayo Clinic, Rochester, MN. 54 - Next-Generation Multitarget Stool DNA Test for Colorectal Cancer Screening: A Prospective Clinical Validation Study (BLUE-C) (Late-Breaking Abstract), ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

