Back
Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 4B - IBD / Obesity / Stomach / Pediatrics
70 - Two-Year Efficacy and Safety of Mirikizumab Following 104 Weeks of Continuous Treatment: Interim Results From the LUCENT-3 Open-Label Extension Study
Wednesday, October 25, 2023
9:20 AM – 9:30 AM PT
Location: Ballroom B

Remo Panaccione, MD
University of Calgary
Calgary, AB, Canada
Presenting Author(s)
Bruce E. Sands, MD, MS, FACG1, Geert D'Haens, MD, PhD2, David B. Clemow, 3, Peter M. Irving, 4, Jordan Johns, PhD3, Theresa Hunter Gibble, PhD, MPH3, Maria T. Abreu, MD5, Scott Lee, 6, Tadakazu Hisamatsu, MD, PhD7, Taku Kobayashi, MD, PhD8, Marla C. Dubinsky, MD9, Séverine Vermeire, MD, PhD10, Corey A. Siegel, MD, MS11, Laurent Peyrin-Biroulet, MD, PhD12, Richard E. Moses, DO, JD3, Joe Milata, 3, Vipin Arora, 3, Remo Panaccione, MD13, Axel Dignass, MD, PhD14
1Icahn School of Medicine at Mount Sinai, New York, NY; 2Amsterdam University Medical Center, Amsterdam, Noord-Holland, Netherlands; 3Eli Lilly and Company, Indianapolis, IN; 4Guy's and St. Thomas’ Hospitals, London, England, United Kingdom; 5University of Miami Miller School of Medicine, Miami, FL; 6Digestive Health Center University of Washington Medical Center, Seattle, WA; 7Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 8Kitasato University Kitasato Institute Hospital, Center for Advanced IBD Research and Treatment, Tokyo, Tokyo, Japan; 9Mount Sinai Kravis Children’s Hospital, New York, NY; 10UZ Leuven, Leuven, Vlaams-Brabant, Belgium; 11Dartmouth-Hitchcock Inflammatory Bowel Disease Center, Dartmouth-Hitchcock Medical Center, Lebanon, NH; 12Last Inserm U954 and CHU de Nancy, Lorraine University, Vandoeuvre-lès-Nancy, Lorraine, France; 13University of Calgary, Calgary, AB, Canada; 14Agaplesion Markus Hospital, Goethe University, Frankfurt, Hessen, Germany
Introduction: Here we present results from the ongoing open-label LUCENT-3 extension study evaluating long-term efficacy and safety of mirikizumab (miri) in patients with moderately-to-severely active ulcerative colitis (UC).
Methods: In LUCENT-3, patients received 200mg miri Q4W subcutaneously. Following 104W of continuous miri treatment, we report clinical response and remission, corticosteroid-free (CSF) remission, endoscopic remission, histologic-endoscopic mucosal improvement (HEMI) and histologic-endoscopic mucosal remission (HEMR), symptomatic remission, bowel urgency clinical meaningful improvement (CMI) and remission scores for patients who were W52 responders and remitters, including induction baseline biologic failure status. Symptom scores for abdominal pain, bowel urgency, stool frequency (SF), and rectal bleeding (RB) from induction baseline were recorded for all patients completing W52. Biologic Failed was defined as prior inadequate response, loss of response, or intolerance to biologic therapy or Janus kinase inhibitors; otherwise, patients were categorized as Not Biologic Failed. Safety data were assessed.
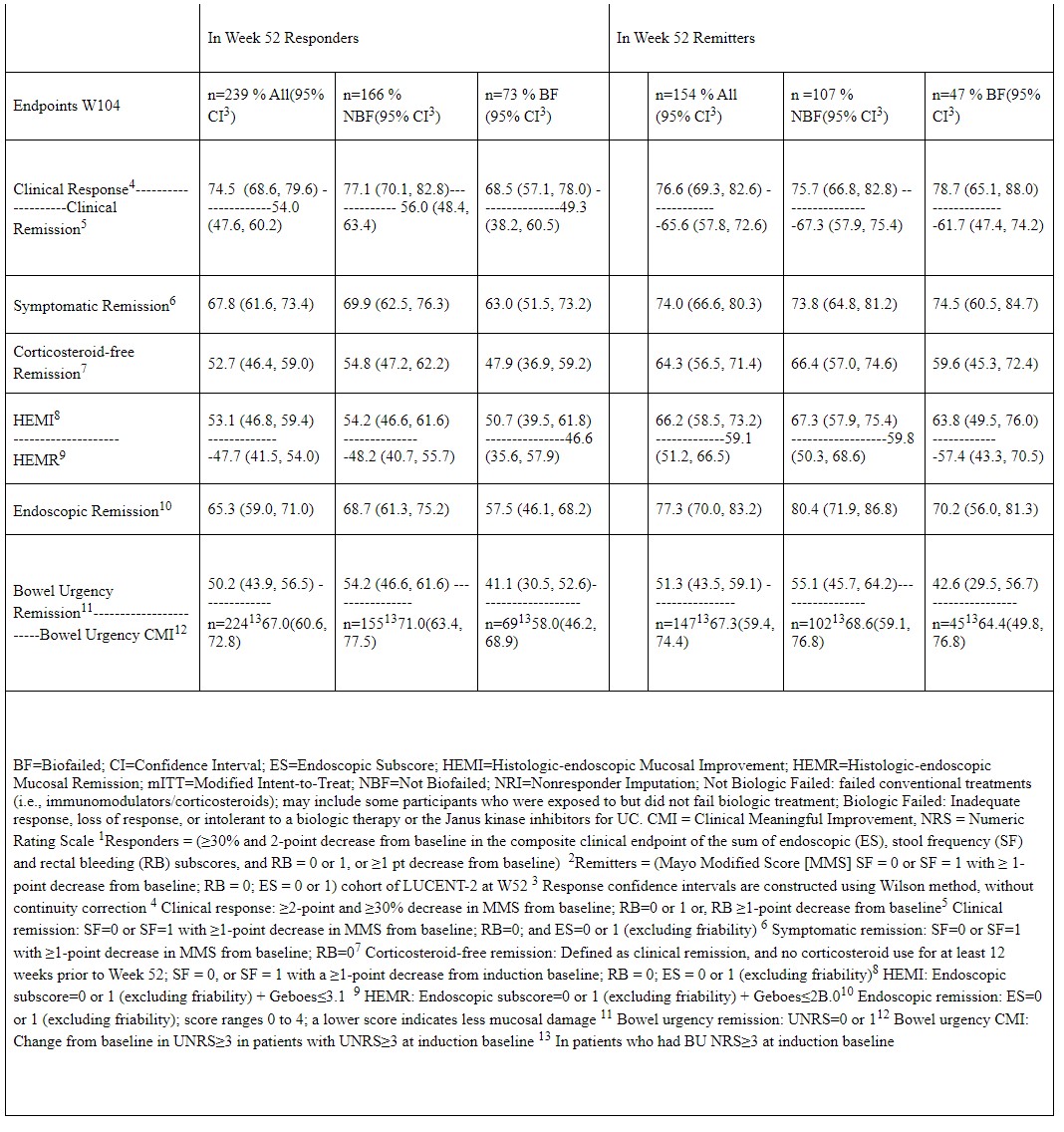
Results: Among W52 miri responders (N=239), 74.5% demonstrated clinical response at W104. Remission rates at W104 for W52 clinical responders were: 54.0% clinical, 52.7% CSF, 65.3% endoscopic, 47.7% HEMR, 67.8% symptomatic, and 50.2% bowel urgency. Patients achieving HEMI and bowel urgency CMI at W104 were 53.1% and 67.0%, respectively. For W52 miri remitters, 76.6% demonstrated clinical response at W104. Remission rates at W104 for W52 clinical remitters (N=154) were: 65.6% clinical, 64.3% CSF, 77.3% endoscopic, 59.1% HEMR, 74.0% symptomatic, and 51.3% bowel urgency. Patients achieving HEMI and bowel urgency CMI at W104 were 66.2% and 67.3%, respectively. Biologic Failed/Non-failed subgroup data were generally similar (Table). Symptom score reductions from induction baseline at W52 were sustained through W104; W52 and W104 scores were respectively: SF: -1.68, -1.79; RB: -1.45, -1.45; bowel urgency: -4.03, -4.44; and abdominal pain: -3.74, -3.91. Severe TEAEs were reported in 4.5% of patients, while 5.2% experienced serious AEs, and 2.8% discontinued treatment due to an AE.
Discussion: These data support the long-term benefit of continuous miri treatment through W104 on clinical, endoscopic, histologic, and symptomatic endpoints, including biologic-failed patients, with no new safety signals identified or deaths reported.
Disclosures:
Bruce E. Sands, MD, MS, FACG1, Geert D'Haens, MD, PhD2, David B. Clemow, 3, Peter M. Irving, 4, Jordan Johns, PhD3, Theresa Hunter Gibble, PhD, MPH3, Maria T. Abreu, MD5, Scott Lee, 6, Tadakazu Hisamatsu, MD, PhD7, Taku Kobayashi, MD, PhD8, Marla C. Dubinsky, MD9, Séverine Vermeire, MD, PhD10, Corey A. Siegel, MD, MS11, Laurent Peyrin-Biroulet, MD, PhD12, Richard E. Moses, DO, JD3, Joe Milata, 3, Vipin Arora, 3, Remo Panaccione, MD13, Axel Dignass, MD, PhD14, 70, Two-Year Efficacy and Safety of Mirikizumab Following 104 Weeks of Continuous Treatment: Interim Results From the LUCENT-3 Open-Label Extension Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Icahn School of Medicine at Mount Sinai, New York, NY; 2Amsterdam University Medical Center, Amsterdam, Noord-Holland, Netherlands; 3Eli Lilly and Company, Indianapolis, IN; 4Guy's and St. Thomas’ Hospitals, London, England, United Kingdom; 5University of Miami Miller School of Medicine, Miami, FL; 6Digestive Health Center University of Washington Medical Center, Seattle, WA; 7Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 8Kitasato University Kitasato Institute Hospital, Center for Advanced IBD Research and Treatment, Tokyo, Tokyo, Japan; 9Mount Sinai Kravis Children’s Hospital, New York, NY; 10UZ Leuven, Leuven, Vlaams-Brabant, Belgium; 11Dartmouth-Hitchcock Inflammatory Bowel Disease Center, Dartmouth-Hitchcock Medical Center, Lebanon, NH; 12Last Inserm U954 and CHU de Nancy, Lorraine University, Vandoeuvre-lès-Nancy, Lorraine, France; 13University of Calgary, Calgary, AB, Canada; 14Agaplesion Markus Hospital, Goethe University, Frankfurt, Hessen, Germany
Introduction: Here we present results from the ongoing open-label LUCENT-3 extension study evaluating long-term efficacy and safety of mirikizumab (miri) in patients with moderately-to-severely active ulcerative colitis (UC).
Methods: In LUCENT-3, patients received 200mg miri Q4W subcutaneously. Following 104W of continuous miri treatment, we report clinical response and remission, corticosteroid-free (CSF) remission, endoscopic remission, histologic-endoscopic mucosal improvement (HEMI) and histologic-endoscopic mucosal remission (HEMR), symptomatic remission, bowel urgency clinical meaningful improvement (CMI) and remission scores for patients who were W52 responders and remitters, including induction baseline biologic failure status. Symptom scores for abdominal pain, bowel urgency, stool frequency (SF), and rectal bleeding (RB) from induction baseline were recorded for all patients completing W52. Biologic Failed was defined as prior inadequate response, loss of response, or intolerance to biologic therapy or Janus kinase inhibitors; otherwise, patients were categorized as Not Biologic Failed. Safety data were assessed.
Results: Among W52 miri responders (N=239), 74.5% demonstrated clinical response at W104. Remission rates at W104 for W52 clinical responders were: 54.0% clinical, 52.7% CSF, 65.3% endoscopic, 47.7% HEMR, 67.8% symptomatic, and 50.2% bowel urgency. Patients achieving HEMI and bowel urgency CMI at W104 were 53.1% and 67.0%, respectively. For W52 miri remitters, 76.6% demonstrated clinical response at W104. Remission rates at W104 for W52 clinical remitters (N=154) were: 65.6% clinical, 64.3% CSF, 77.3% endoscopic, 59.1% HEMR, 74.0% symptomatic, and 51.3% bowel urgency. Patients achieving HEMI and bowel urgency CMI at W104 were 66.2% and 67.3%, respectively. Biologic Failed/Non-failed subgroup data were generally similar (Table). Symptom score reductions from induction baseline at W52 were sustained through W104; W52 and W104 scores were respectively: SF: -1.68, -1.79; RB: -1.45, -1.45; bowel urgency: -4.03, -4.44; and abdominal pain: -3.74, -3.91. Severe TEAEs were reported in 4.5% of patients, while 5.2% experienced serious AEs, and 2.8% discontinued treatment due to an AE.
Discussion: These data support the long-term benefit of continuous miri treatment through W104 on clinical, endoscopic, histologic, and symptomatic endpoints, including biologic-failed patients, with no new safety signals identified or deaths reported.

Table: Table. LUCENT-3 Response and Remission at 104 Weeks of Continuous Treatment in LUCENT-2 Responders1 and Remitters2 - mITT Population, NRI
Disclosures:
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, Speaker’s fees. Adiso Therapeutics – Consultant. Alimentiv – Consultant. Amgen – Consultant. Arena pharmaceuticals – Consultant. Artizan Biosciences – Consultant. Artugen Therapeutics – Consultant. AstraZeneca – Consultant. Bacainn Therapeutics – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, speaking fees and other support. Calibr – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Connect Biopharm – Consultant. Cytoki Pharma – Consultant. Eli Lilly – Consultant, speaking fees and other support. Enthera – Consultant. Evommune – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Galapagos – Consultant. Genentech – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant. HMP Acquisition – Consultant. Imhotex – Consultant. Immunic – Consultant. InDex Pharmaceuticals – Consultant. Innovation Therapeutics – Consultant. Inotrem – Consultant. Ironwood Pharmaceuticals – Consultant. Janssen – Grant/Research Support, consulting and speaking fees and other support. Johnson & Johnson – Consultant. Kaleido Biosciences – Consultant. Kallyope – Consultant. Merck – Consultant. MiroBio – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. OSE Immunotherapeutics – Consultant. Pfizer Inc – Consultant, Grant/Research Support, speaking fees and other support. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. RedHill Biopharma – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Synlogic Operating Company – Consultant. Takeda – Grant/Research Support, consulting and speaking fees and other support. Target RWE – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. USWM Enterprises – Consultant. Ventyx Biosciences – Consultant, personal fees and stock options for consulting. Viela Bio – Consultant.
Geert D'Haens: AbbVie – Consultant, Speakers Bureau. Alimentiv – Consultant, Speakers Bureau. Boehringer Ingelheim – Consultant, Speakers Bureau. Cellitrion – Consultant, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Galapagos – Consultant, Speakers Bureau. GlaxoSmithKline – Consultant, Speakers Bureau. Gossamerbio – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Lilly – Consultant, Speakers Bureau. MSD – Consultant, Speakers Bureau. Pfizer – Consultant, Speakers Bureau. Progenity – Consultant, Speakers Bureau. Prometheus Biosciences – Consultant, Speakers Bureau. Prometheus Laboratories – Consultant, Speakers Bureau. Takeda – Consultant, Speakers Bureau. Tillotts – Consultant, Speakers Bureau.
David B. Clemow: Eli Lilly and Company – Employee, Speakers Bureau.
Peter M. Irving indicated no relevant financial relationships.
Jordan Johns: Eli Lilly and Company – Employee, stockholder.
Theresa Hunter Gibble: Eli Lilly and Company – Employee, stockholder.
Maria T. Abreu: AbbVie – Advisory Committee/Board Member, Consultant. Alimentiv – Speakers Bureau. Amgen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Arena – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant. Celsius Therapeutics – Advisory Committee/Board Member, Consultant. Eli Lilly – Advisory Committee/Board Member, Consultant. Gilead Sciences – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Microba Life Sciences, – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant. Prometheus Biosciences – Advisory Committee/Board Member, Consultant. Takeda – Speakers Bureau. UCB Biopharma – Advisory Committee/Board Member, Consultant. WebMD Global – Advisory Committee/Board Member, Consultant.
Scott Lee: AbbVie, Applied Molecular Transport, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly and Company, Janssen and Protagonist Therapeutics – Consultant, Grant/Research Support.
Tadakazu Hisamatsu: AbbVie GK – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Alfresa Pharma Corporation and EA Pharma Co., Ltd – Grant/Research Support. Daiichi-Sankyo – Grant/Research Support. EA Pharma Co, Ltd – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Eli Lilly – Consultant. Gilead Sciences – honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Janssen Pharmaceutical K.K. – honoraria and had expenses paid to attend or give a presentation/advice at a meeting. JIMRO Co., Ltd – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. KISSEI PHARMACEUTICAL CO., LTD – honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Kyorin Pharmaceutical Co., Ltd – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Mitsubishi Tanabe Pharma Corporation – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Mochida Pharmacuetical Co., Ltd – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Nichi-Iko Pharmaceutical Co., Ltd – honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Nippon Kayaku Co., Ltd – Grant/Research Support. Pfizer Japan Inc. – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Takeda Pharmaceutical Co., Ltd – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Zeria Pharmaceutical Co., Ltd – Grant/Research Support.
Taku Kobayashi: , Nippon Kayaku, Otsuka, Pfizer Japan K.K., Takeda and Zeria Pharmaceutical – Grant/Research Support. ., Takeda, Thermo Fisher Scientific and Zeria Pharmaceutical – Speakers Bureau. – Bristol Myers Squibb, EA Pharma, Eli Lilly and Company and Janssen – Advisory Committee/Board Member. AbbVie, ActivAid, Alfresa Pharma, Bristol Myers Squibb, EA Pharma, Eli Lilly Japan K.K., Gilead Sciences, Google Asia Pacific – Grant/Research Support. AbbVie, ActivAid, Alfresa Pharma, EA Pharma, Janssen Pharmaceutical K.K., Kissei, Kyorin, Mitsubishi Tanabe Pharma, – Payment for expert testimony. AbbVie, ActivAid, Alfresa Pharma, Galapagos NV, Janssen Pharmaceutical K.K., JIMRO, Kyorin, Mitsubishi Tanabe Pharma,
Nippon Kayaku, Pfizer Japan K.K – Speakers Bureau. Janssen Pharmaceutical K.K., JIMRO, JMDC,
Kyorin, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, – Grant/Research Support. Mochida Pharmaceutical, Nippon Kayaku,
Pfizer Japan K.K. and Takeda – Payment for expert testimony.
Marla Dubinsky: AbbVie – Consultant, Grant/Research Support. Arena Pharmaceuticals – Consultant. Astra Zeneca – Consultant. Celgene – Consultant. Genentech Inc. – Consultant. Gilead Sciences – Consultant. Janssen – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant, Grant/Research Support. Prometheus Biosciences – Consultant, Grant/Research Support. Prometheus Labs – Consultant, Grant/Research Support. Takeda – Consultant, Licensing fees. Thabor – Consultant. Trellus Health – Stock-publicly held company(excluding mutual/index funds). UCB Pharma – Consultant.
Séverine Vermeire: AbbVie – Consultant, Grant/Research Support. AbolerIS Pharma – Grant/Research Support. AgomAb – Grant/Research Support. Alimentiv – Consultant. Arena Pharmaceuticals – Consultant. AstraZeneca – Consultant. Avaxia Biologics – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. CVasThera – Consultant, Speakers Bureau. Cytoki Pharma – Consultant, Speakers Bureau. Dr. Falk Pharma – Consultant, Speakers Bureau. Eli Lilly – Consultant, Speakers Bureau. Ferring Pharmaceuticals – Consultant, Speakers Bureau. Galapagos – Consultant, Grant/Research Support, Speakers Bureau. Genentech/Roche – Consultant, Speakers Bureau. Gilead – Consultant, Speakers Bureau. GSK – Consultant, Speakers Bureau. Hospira – Consultant, Speakers Bureau. IMIDomics – Consultant, Speakers Bureau. Janssen Pharmaceuticals – Consultant, Speakers Bureau. Johnson & Johnson – Consultant, Grant/Research Support, Speakers Bureau. Materia Prima – Consultant, Speakers Bureau. MiroBio – Consultant, Speakers Bureau. Morphic – Consultant, Speakers Bureau. MRM Health – Consultant, Speakers Bureau. MSD – Consultant, Speakers Bureau. Mundipharma – Consultant, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. ProDigest – Consultant, Speakers Bureau. Progenity – Consultant, Speakers Bureau. Prometheus – Consultant, Speakers Bureau. Robarts Clinical Trials – Consultant, Speakers Bureau. Second Genome – Consultant, Speakers Bureau. Shire – Consultant, Speakers Bureau. Surrozen – Consultant, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau. Theravance Biopharma – Consultant, Speakers Bureau. Tillotts Pharma – Consultant, Speakers Bureau. Zealand Pharma – Consultant, Speakers Bureau.
Corey Siegel: Abbvie – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. BMS – Advisory Committee/Board Member, Consultant. Eli Lilly – Advisory Committee/Board Member, Consultant. Fresnius – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant, Grant/Research Support, Consultant/advisory board, speaker for CME activities. Napo Pharmaceuticals – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Grant/Research Support, speaker for CME activities. Prometheus Biosciences – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau.
Laurent Peyrin-Biroulet: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Allergan – personal fees. Alma – personal fees. Amgen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Applied Molecular Transport – personal fees. Arena – personal fees. Biogaran – Advisory Committee/Board Member, Consultant, Speakers Bureau. Biogen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant, Speakers Bureau. Bristol Myers Squibb – personal fees. Celgene – Advisory Committee/Board Member, Consultant, Speakers Bureau. Celltrion – Advisory Committee/Board Member, Consultant, Speakers Bureau. CTMA – Stock Options. Eli Lilly – Advisory Committee/Board Member, Consultant, Speakers Bureau. Enterome – personal fees. Enthera – personal fees. Ferring – Advisory Committee/Board Member, Consultant, Speakers Bureau. Forward Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Fresenius – personal fees. Genentech – Advisory Committee/Board Member, Consultant, Speakers Bureau. Gilead – personal fees. H.A.C. Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Hikma – personal fees. Hospira/Pfizer – Advisory Committee/Board Member, Consultant, Speakers Bureau. Index Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speakers Bureau. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Lycera – Advisory Committee/Board Member, Consultant, Speakers Bureau. Merck – Advisory Committee/Board Member, Consultant, Speakers Bureau. Mitsubishi – Advisory Committee/Board Member, Consultant, Speakers Bureau. MSD – Grant/Research Support, personal fees. Mylan – personal fees. Nestlé – personal fees. Norgine – Advisory Committee/Board Member, Consultant, Speakers Bureau. Oppilan Pharma – personal fees. OSE Immunotherapeutics – personal fees. Pfizer – personal fees. Pharmacosmos – personal fees. Roche – personal fees. Samsung Bioepis – Advisory Committee/Board Member, Consultant, Speakers Bureau. Sandoz – Advisory Committee/Board Member, Consultant, Speakers Bureau. Sterna – personal fees. Sublimity Therapeutics – personal fees. Takeda – Advisory Committee/Board Member, Consultant, Speakers Bureau. Theravance – Advisory Committee/Board Member, Consultant, Speakers Bureau. Tillots – Advisory Committee/Board Member, Consultant, Speakers Bureau. Vifor – Advisory Committee/Board Member, Consultant, Speakers Bureau.
Richard E. Moses: Eli Lilly and Company – Employee, Stock-privately held company.
Joe Milata: Eli Lilly and Company – Consultant, Stock Options.
Vipin Arora: Eli Lilly and Company – Employee, Stock Options.
Remo Panaccione: Abbivax – Consultant. Abbott – Consultant. AbbVie – Consultant. Alimentiv – Consultant. Amgen – Consultant. Arena – Consultant. AstraZeneca – Consultant. Biogen – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. Celltrion – Consultant. Cosmos Technology – Consultant. Eisai – Consultant. Elan – Consultant. Eli Lilly – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Galapagos – Consultant. Genentech – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. JAMP Bio – Consultant. Janssen – Consultant. Merck – Consultant. Mylan – Consultant. Novartis – Consultant. Oppilan Pharma – Consultant. Organon – Consultant. Pandion Pharma – Consultant. Pendopharm – Consultant. Pfizer Inc – Consultant. Progenity – Consultant. Prometheus – Consultant. Protagonist Therapeutics – Consultant. Roche – Consultant. Sandoz – Consultant. Satisfai Health – Consultant. Shire – Consultant. Sublimity Therapeutics – Consultant. Takeda Pharmaceuticals – Consultant. Theravance Biopharma – Consultant. Trellus – Consultant. UCB – Consultant. Ventyx – Consultant. Viatris – Consultant.
Axel Dignass: AbbVie – Consultant, participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. Abivax – participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees. Amgen – Consultant. Arena Pharmaceuticals – Consultant, participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees. Biogen – Consultant, Speakers Bureau. Boehringer Ingelheim – Consultant. Bristol Myers Squibb/Celgene – Consultant, participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees. CED Service GmbH – Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Dr Falk Foundation – Consultant, participation in clinical trials, review activities and manuscript preparation, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Fresenius Kabi – Consultant. Galapagos – Consultant, participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. Gilead – participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. High5MD – Speakers Bureau. Janssen – Consultant, participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. Lilly – Consultant. Materia Prima – Speakers Bureau. MedToday – Speakers Bureau. MSD – Consultant, Speakers Bureau. Pfizer – Consultant, participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. Pharmacosmos – Consultant. Roche/Genentech – Consultant. Sandoz/Hexal – Consultant. Streamed-Up – Speakers Bureau. Takeda – Consultant, manuscript preparation, Speakers Bureau. Thieme – manuscript preparation. Tillotts – Consultant, Speakers Bureau. UniMed Verlag – manuscript preparation. Vifor Pharma – Consultant, Speakers Bureau.
Bruce E. Sands, MD, MS, FACG1, Geert D'Haens, MD, PhD2, David B. Clemow, 3, Peter M. Irving, 4, Jordan Johns, PhD3, Theresa Hunter Gibble, PhD, MPH3, Maria T. Abreu, MD5, Scott Lee, 6, Tadakazu Hisamatsu, MD, PhD7, Taku Kobayashi, MD, PhD8, Marla C. Dubinsky, MD9, Séverine Vermeire, MD, PhD10, Corey A. Siegel, MD, MS11, Laurent Peyrin-Biroulet, MD, PhD12, Richard E. Moses, DO, JD3, Joe Milata, 3, Vipin Arora, 3, Remo Panaccione, MD13, Axel Dignass, MD, PhD14, 70, Two-Year Efficacy and Safety of Mirikizumab Following 104 Weeks of Continuous Treatment: Interim Results From the LUCENT-3 Open-Label Extension Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

